Over the past three years, SIAPS has expanded the use of RxSolution to a total of 350 sites across South Africa. SIAPS is also developing numerous customized modules and modifications to meet the specialized needs of different clinical settings including expanded functionality for barcode tracking, biometric monitoring, and mobile phone applications.
SIAPS is working to help governments, hospitals, and health facilities better manage data by strengthening the systems that support effective pharmaceutical information management. In addition to improving the collection, quality, and analysis of data, SIAPS is working in partnership with local stakeholders to develop informational dashboards which highlight and make available the key pieces of data needed for effective decision making.
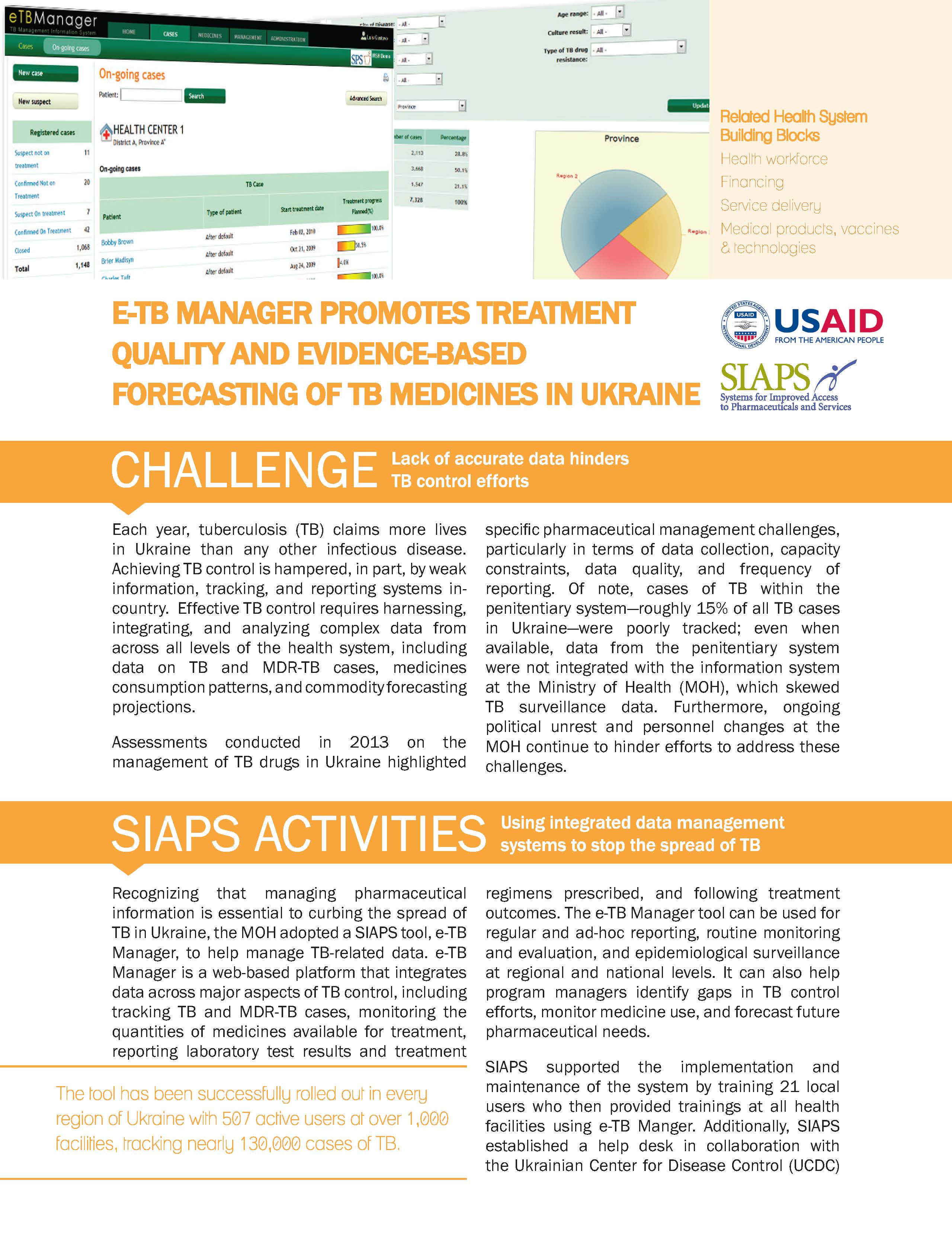
Assessments conducted in 2013 on the management of TB drugs in Ukraine highlighted specific pharmaceutical management challenges, particularly in terms of data collection, capacity constraints, data quality, and frequency of reporting. Of note, cases of TB within the penitentiary system―roughly 15% of all TB cases in Ukraine―were poorly tracked; even when available, data from the penitentiary system were not integrated with the information system at … Read more
A number of low resource countries are facing a severe and prolonged shortage of health workers, particularly in the pharmaceutical sector where pharmacists, pharmacy assistants, and technicians are becoming especially scarce. With treatment programs, such as those for HIV/AIDS and TB, expanding in many countries, more pharmacists and pharmacy assistants are required to provide effective services. Additionally, overstretched pharmacists and other healthcare workers are often … Read more
An assessment of Namibia’s pharmaceutical system conducted in 2009 identified a number of capacity building challenges to be addressed to improve pharmaceutical services. These challenges included shortages of key staff, unmanageable workloads, and inadequate storage space for medicines. In response, the Ministry of Health and Social Services (MOHSS) identified several key mechanisms to address these challenges and strengthen the pharmaceutical sector including improving the functionality … Read more
SIAPS technical support has helped strengthen the capacity of the national registration committee and streamline medicines registration. As a result, the number of registered medicines has increased from 200 in 2010 to over 3,000 in 2014; 72% of the medicines included on DRC’s essential medicines list currently have at least one product registered, up from 44% in 2011. The backlog of applications has … Read more
Only with a holistic, systems-level approach can policies, procedures, and other guidance documents provide the direction, integration, and coordination required to meet the unique pharmaceutical needs of a country. Working with in-country partners, SIAPS helps to analyze each unique country context, identify priority issues, harmonize approaches, and develop well-informed and locally appropriate policy documents to support good governance, which help to strengthen the performance … Read more
The weak regulatory system in Swaziland poses a threat to public health and safety and has been an obstacle in the country’s effort to improve access to quality essential medicines and services for managing HIV, controlling tuberculosis, and delivering other priority public health interventions. The legislation governing the regulation of medicines, pharmaceutical establishments, and the pharmacy profession in Swaziland dates back to 1929 and … Read more
By publishing this newsletter, The Directorate General of Drug Administration (DGDA) will be able to communicate information on the safety of medicines and other health products to health care professionals, consumers, and the public on a regular basis. In the event of an emergency, when serious risks arise, that information will become crucial to ensuring … Read more
The Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program has designed and implemented a certificate course to ensure the strengthening and sustainability of rational medicine use in the Dominican Republic. Universidad Central del Este (UCE) conducted the first course to thirty-two students in mid-2016 and shall conduct a second course in the last … Read more