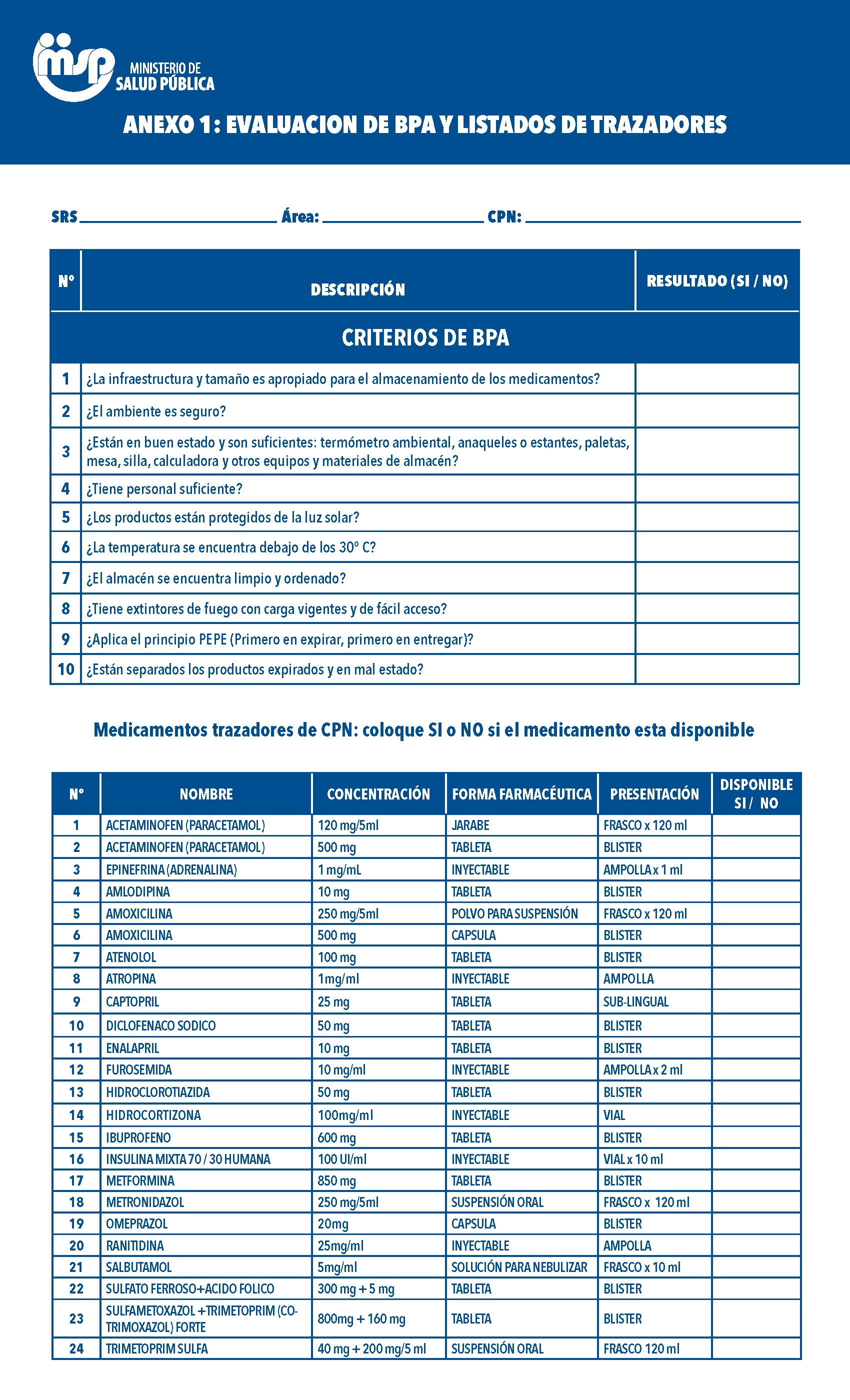
Formulario de inventario para la evaluación de las Buenas Prácticas de Almacenamiento (BPA) y listados de trazadores en Centros de primer nivel de atención (CPN), Centros Especializados de Atención en Salud (CEAS) y medicamentos trazadores de VIH/SIDA en CEAS.
Chronic underfunding of the health sector coupled with long-term civil unrest in the Democratic Republic of the Congo (DRC) has contributed to insufficient regulatory capacity to effectively manage the registration and approval of new medicines in the country. In partnership with the USAID-funded SIAPS Program, the country’s Ministry of Health supported a number of broad … Read more
The National TB Control Program (NTP) in the Philippines is continuously scaling up its operations in the diagnosis and treatment of TB to achieve the results and deliverables described in the 2010–2016 Philippine Plan of Action to Control Tuberculosis. The USAID-funded SIAPS Program is supporting the NTP in its effort to increase the capacity of various … Read more
The availability and quality of HIV commodity, including antiretrovirals and HIV test kits, increases the demand for HIV care services and enables the scale-up of antiretroviral therapy. In West Africa and Central Africa, stock-outs often occurred because of poor coordination and information sharing among partners and lack of a reliable early warning system. With funding … Read more
Burkina Faso, Cameroon, Central Africa, Guinea, HIV/AIDS, J Adu, JB Evi, Niger, OSPSIDA, Supply chain management, Technical Report, Togo, West Africa Regional Project
Inefficient and irrational use of medicines is a well-documented problem in both developed and developing countries. It leads to cost increases and adverse clinical effects for patients. The inappropriate use of medicine can be reduced if health care professionals involved in the different aspects of medicine use promote good practices for medicine management and use. … Read more
USAID’s West African office asked SIAPS to provide support to six countries in the West and Central African region—Burkina Faso, Benin, Cameroon, Guinea, Niger, and Togo—to establish a web-based regional dashboard (OSPSIDA.org) that will create an early warning system (EWS) to monitor HIV and AIDS commodities and to detect and minimize the risk of stock-out … Read more
Benin, Cameroon, dashboard, Guinea, HIV/AIDS, J Adu, JB Evi, M Islam, Mali, Niger, OSPSIDA, S Doumbia, Supply chain management, Technical Report, Togo, West Africa Regional Project
Developing a CMS strategic plan can help align pharmaceutical supply chain objectives with overall public-sector health supply chain strategies. It can help ensure that health commodities are readily available to health facilities through an uninterrupted supply chain; minimize waste and losses; improve business and financial growth; and respond to changes in the supply chain, such … Read more
South Sudan’s health system is struggling to overcome a myriad of challenges, including poor pharmaceutical supply management practices, weak infrastructure, and inadequate skilled manpower. The outbreak of civil unrest in the nascent nation in December 2013 further exacerbated the already dire situation. South Sudan has one of the highest maternal mortality rates in the world, … Read more
MSH has implemented pharmaceutical management projects in Ethiopia since 2005, through the Rational Pharmaceutical Management Plus Project and the Strengthening Pharmaceutical Systems (SPS) Program and most recently the Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program. The SIAPS Program (2011 to 2016) aims to reduce morbidity and mortality, primarily for HIV and AIDS, … Read more
In 2012, the Government of Mozambique began a national accelerated response to HIV and AIDS. As a result, better awareness, funding, and deployment of proven interventions have significantly improved HIV prevention, treatment, and care support. This includes the rapid scaling up of antiretroviral therapy. With significant support from the US President’s Emergency Plan for AIDS … Read more