SIAPS and its predecessor programs have assisted numerous countries in strengthening governance to promote robust decision making, enhance accountability, reduce opportunities for corruption, and improve efficiencies to enable better access to and use of quality-assured medicines. This compendium draws on these experiences and provides a collection of examples of strategies and approaches for strengthening governance … Read more
Bangladesh, Cameroon, Democratic Republic of Congo, Ethiopia, governance, H Walkowiak, Philippines, S Putter, Sierra Leone, Swaziland, T Hafner, Ukraine
A multicountry user experience analysis of e-TB Manager and an in-depth study in Ukraine were published. However, the procedural aspects of e-TB Manager implementation in each country were not documented. While facilitators and barriers for eHealth implementation in resource-constrained settings are well known, the objective of this paper is to summarize the tailored implementation approaches given local context, which … Read more
Armenia, Azerbaijan, Bangladesh, Brazil, Cambodia, e-TB Manager, Indonesia, K Sawyer, N Konduri, Namibia, Nigeria, Technical Report, tuberculosis, Ukraine, Vietnam
Project dates: September 2012 – June 2017
The availability of a unified essential medicines list (EML) with evidence-based clinical efficacy to be used by the Ministry of Health (MOH) for the state-guaranteed package of services is an essential part of the successful launch of the health care reform initiative in Ukraine. This required the development and institutionalization of a process to ensure … Read more
On June 30, 2017, SIAPS closed its office in Ukraine. Since 2011, SIAPS worked with the Ministry of Health (MOH) and other stakeholders strengthening the country’s pharmaceutical systems and enhancing transparency of procurement practices and rational medicine use. SIAPS provided assistance to streamline medicine selection by harmonizing the essential medicines list (EML). This was done … Read more
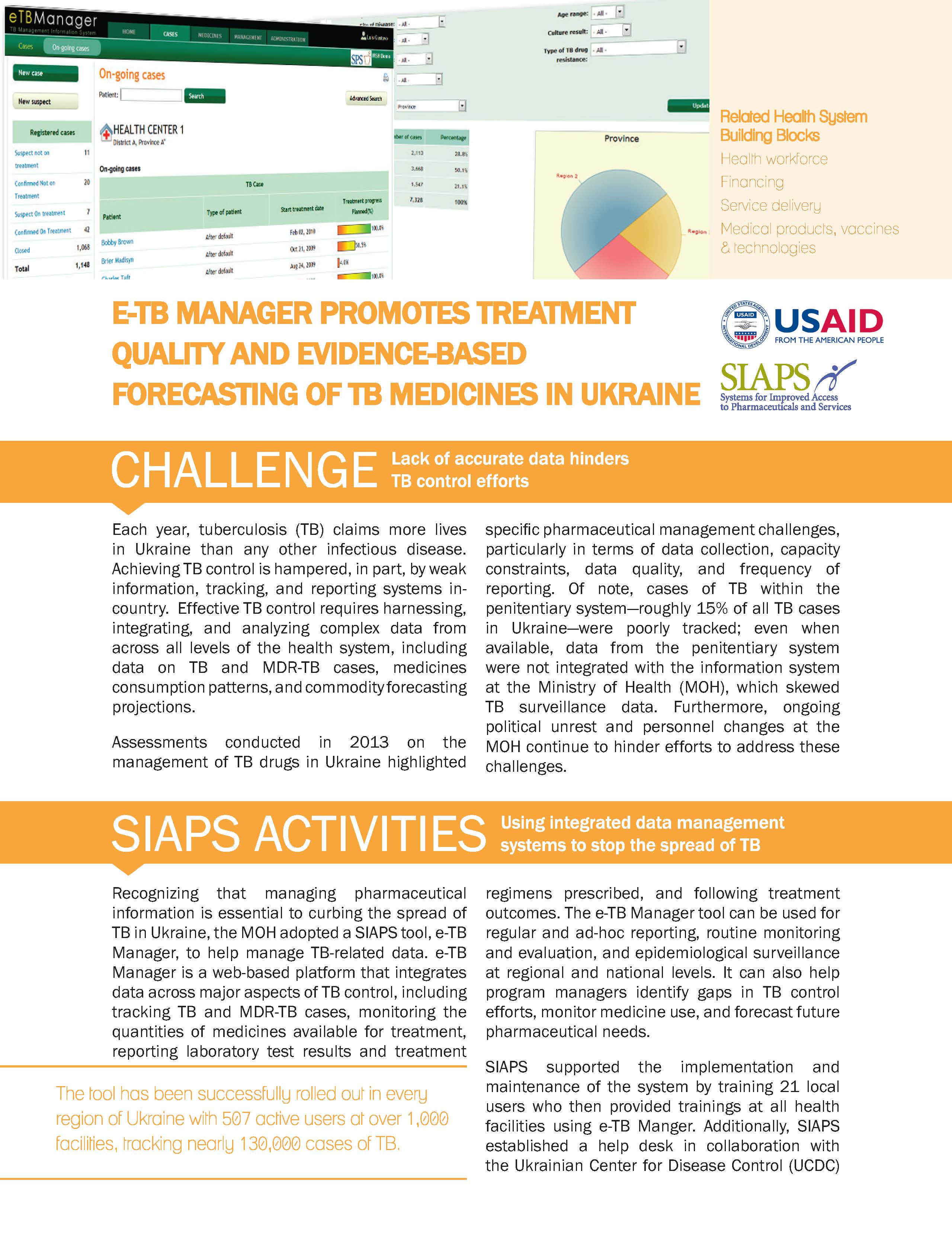
Tuberculosis, which is the leading cause of death among infectious diseases in Ukraine, is especially dangerous due to the high estimated number of patients with multidrug-resistant TB. Since its implementation in 2008, e-TB Manager, a digital case management tool, has shown strong results: 81% of users, including doctors, nurses, and laboratory professionals, agree that it … Read more
Assessments conducted in 2013 on the management of TB drugs in Ukraine highlighted specific pharmaceutical management challenges, particularly in terms of data collection, capacity constraints, data quality, and frequency of reporting. Of note, cases of TB within the penitentiary system―roughly 15% of all TB cases in Ukraine―were poorly tracked; even when available, data from the penitentiary system were not integrated with the information system at … Read more
Konduri N, Lebega O. Analysis of medicines expenditure in ukraine: Implications for rational selection. E9 Short oral presentation, Part 2a (Practice & Education) at the 76th FIP World Congress of Pharmacy and Pharmaceutical Sciences 2016, Buenos Aires, Argentina. August 30, 2016.
In Ukraine in recent years, failed rounds of centralized public tenders for pharmaceuticals and blockage of public funds in antimonopoly litigations were regarded as a harbinger of the impending crisis in the public health sector. The Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program was requested to step in and provide technical assistance … Read more
When doctors and patients fail to adhere to proper standards of treatment, it results in a higher disease burden, including greater financial costs, mortality, and morbidity. In Ukraine, the need to encourage better adherence to standard treatment guidelines became clear in 2011, when a study by SIAPS Program’s predecessor, the Strengthening Pharmaceutical Systems (SPS) Program, … Read more