For the French version of this article, please click here. For six years, the USAID-funded Systems for Improved Access to Pharmaceuticals and Services (SIAPS) program has been working with Mali’s Ministry of Health and Public Hygiene (MSHP) to enhance governance and transparency in the pharmaceutical sector, strengthen the supply chain system for pharmaceutical commodities, and … Read more
Archive Where We Work
Le Mali célèbre les six ans d’activité du programme des systèmes pour l’amélioration de l’accès aux produits et services pharmaceutiques
Pendant six ans, le programme des systèmes pour l’amélioration de l’accès aux produits et services pharmaceutiques (SIAPS), financé par l’Agence des Etats-Unis pour le Développement Internationale (USAID) a apporté son soutien pour améliorer la gouvernance et la transparence dans le secteur pharmaceutique, fournir une assistance technique afin de développer des outils de gestion pharmaceutique et … Read more
Setting the stage for pharmaceutical system reform in Ukraine
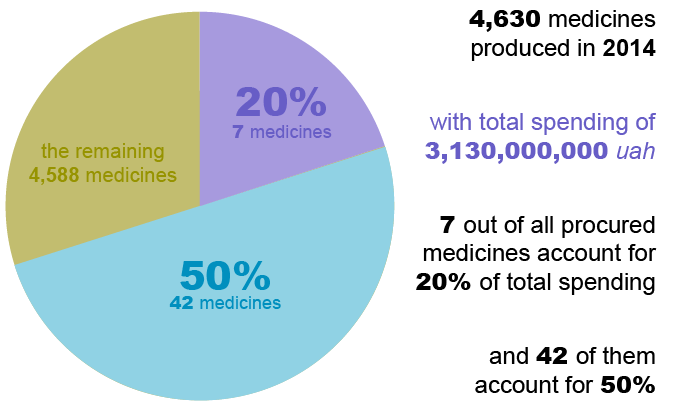
By Juanita Folmsbee, Ukraine Country Project Director, SIAPS and SAFEMed To be fully effective, health system strengthening projects should have sustainable impact and lay the groundwork for future progress. Here’s how SIAPS’ work supported health system reform in Ukraine. SIAPS worked in Ukraine for four years, from 2013 through 2017. Ukraine has the most severe … Read more
Lucky Specials Brings Focus to TB Awareness Week in Namibia
Theresia Cloete was diagnosed with tuberculosis (TB) in 2002. She was put on treatment but after four months stopped taking her TB medication. Last year, she was diagnosed with drug-resistant TB. Like Cloete, many TB patients stop taking their life-saving medication before they are cured. She is now one of eight in-patients waiting for stabilization … Read more
Rapid Evaluation of the Medicines Registration System in Benin

The director of the Department of Pharmacy, Medicines, and Diagnostics (DPMED) wishes to adopt suitable software to strengthen the registration system for medicines and other health products. Although computerization yields improvements in the management of regulatory information, its effectiveness will largely depend on the presence of adequate medicines registration procedures and the system’s overall compliance with … Read more
Quality and Safety of HIV/AIDS Medicines in Namibia October 2015-September 2016

In the last five years, SIAPS has provided technical assistance to the Namibia Medicines Regulatory Council (NMRC) to strengthen its capacity for registration and quality testing by training technical staff in medicines dossier evaluation, developing guidelines for conducting the routine PMS of quality of medicines, and collecting medicine samples at selected ART and TB treatment sites … Read more
SIAPS Angola End of Project Report

Project dates: September 2012 – September 2016
Strengthening the Supply Chain Governance Framework for Pharmaceuticals and Health Products in the Philippines – Technical Brief

The Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Project, funded by the US Agency for International Development, helps countries promote access to safe, quality, and cost-effective pharmaceuticals and health commodities by using a system strengthening approach that engages stakeholders, builds and strengthens existing health systems, or establishes a new one, if necessary. SIAPS … Read more
Strengthening the Supply Chain Governance Framework for Pharmaceuticals and Health Products in the Philippines

The Department of Health (DOH) is ultimately responsible and accountable for ensuring that Filipinos have access to quality health services. An effective supply chain is essential for DOH to ensure that lifesaving health products are available, accessible, and effectively used for clients. Recently, the DOH has shown its commitment by creating the Supply Chain Management Unit … Read more
Philippines Pharmacovigilance Training

SIAPS conducted a training on pharmacovigilance (PV) to increase the capacity of the National Tuberculosis Program, the Lung Center of the Philippines, Pharmaceutical Division, and Food and Drug Administration. Strengthening the capacity of staff in these organizations and other stakeholders in this area of PV reinforces current safe scale-up efforts and introduction of these lifesaving … Read more


