The absence of regulatory systems to monitor the quality, safety, and efficacy of medicines can compromise the overall effectiveness of health care services and endanger the public health. A strong regulatory system is considered an essential component of a health system. In Ethiopia, the Food, Medicine and Health Care Administration and Authority (FMHACA), formerly known … Read more
All medicines carry some risk of adverse events; although certain risks are identified when medicines are tested during clinical trials, others aren’t recognized until after the medicine is on the market and has been used in “real world” settings. Adverse events not only endanger the health of patients, but if not well managed, they may … Read more
Despite an increase in access to medicines in low- and middle-income countries (LMICs), fully functional pharmacovigilance and regulatory systems are not yet in place. Strengthening regulatory and pharmacovigilance systems is a global imperative for preventing harm and improving outcomes in treatment and prevention programs. The Asia region both supplies and purchases medical products. A better … Read more
In this issue: National PV Program Launched, Workshop on Condemnation of Unusable and Obsolete Items, Workshop to Finalize the Procurement Operations Manual, Monthly DGFP Procurement Meeting, De-Junking of Obsolete Items from the Family Planning Central Warehouse, SIAPS Hands Over Stock Status Reporting System to the DGFP, SOPs for Supply Chain Management of TB Drugs and Supplies, Workshop to Develop a Standard Bidding … Read more
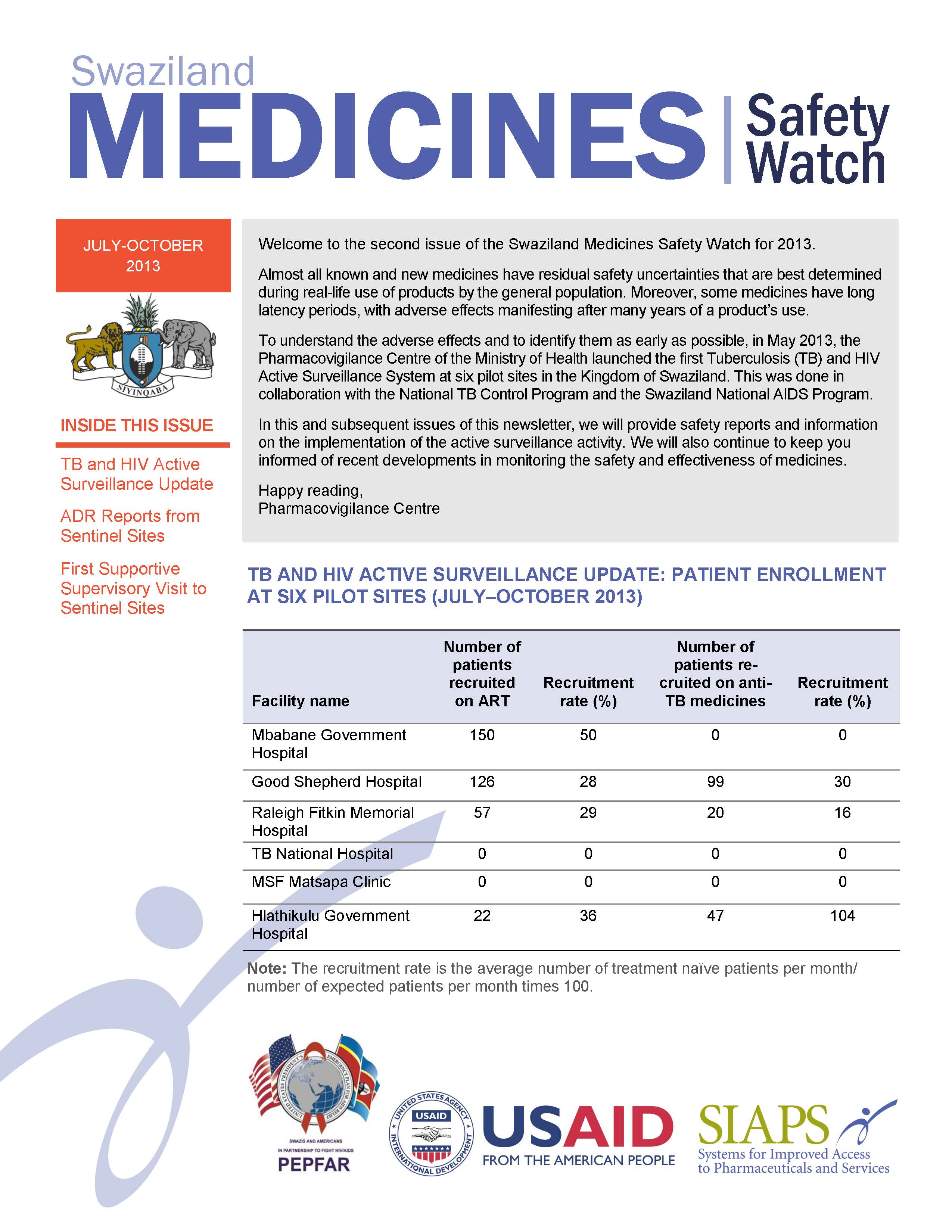
In collaboration with the National TB Control Program and the National AIDS Program, Swaziland’s Pharmacovigilance Centre launched the first Tuberculosis (TB) and HIV Active Surveillance System at six pilot sites in May 2013. The Swaziland Medicines Safety Watch provides safety reports and information on the implementation of the active surveillance activity.
The SIAPS/SCMS/BLC Namibia e-Newsletter is a monthly newsletter that keeps you abreast of activities fundedby the USAID and implemented by MSH Namibia. Key focus areas are strengthening health systems, capacity building, and human resource development.
Quarterly Pharmacovigilance Bulletin describing the work being done in the Democratic Republic of the Congo with the Centre National de Pharmacovigilance.
The overall goal of the SIAPS/Bangladesh program is to build the capacity of MOHFW and its key directorates—DGFP, DGHS, DGDA, and HED—and other indigenous institutions to efficiently and effectively manage their procurement and supply chain management activities. Special focus will be given to TB commodity management at all levels. Read about their progress toward this … Read more
Download The overall goal of the SIAPS/Bangladesh program is to build the capacity of MOHFW and its key directorates—DGFP, DGHS, DGDA, and HED—and other indigenous institutions to efficiently and effectively manage their procurement and supply chain management activities. Special focus will be given to TB commodity management at all levels. Read about their progress toward … Read more
Download The purpose of this document is to guide stakeholders, such as faculty and staff in the fields of medicine, pharmacy, nursing, and public health, through the process of integrating RMU-related content into their preservice training curricula for medical, nursing, pharmacy, or public health students.