A pharmacovigilance system, through active surveillance in sentinel sites, is proposed to monitor the safety and tolerability of antiretroviral medicines (ARV) and anti-tuberculosis (TB) medicines at antiretroviral treatment (ART) clinics and TB clinics in Swaziland. The goal of this activity is to develop, implement, and demonstrate the local feasibility of a practical and sustainable pharmacovigilance … Read more
A key element of successful tuberculosis (TB) control programs is adherence to treatment. Non-adherence results in increased length and severity of illness, death, disease transmission, and drug resistance. The purpose of this study was to estimate the morbidity and mortality impact and economic costs of non-adherence to TB medicines resulting from treatment interruption due to … Read more
One of the key elements of successful tuberculosis (TB) control programs is adherence to treatment, which is a cornerstone of most international and national policies and guidelines. Non-adherence results in increased length and severity of illness, death, disease transmission, and drug resistance. Treatment interruption is often due to patient-related factors—classed as loss to follow-up (LTFU)—but … Read more
One of the key elements of successful tuberculosis (TB) control programs is adherence to treatment, which is a cornerstone of most international and national policies and guidelines. Non-adherence results in increased length and severity of illness, death, disease transmission, and drug resistance. Treatment interruption is often due to patient-related factors—classed as loss to follow-up (LTFU)—but … Read more
This guide is intended to serve as a reference for national-level quantification, forecasting, and supply planning to inform the procurement of anti-TB medicines in the Philippines. It provides practical guidance for program managers, technical staff, and other key personnel, outlining common considerations and best practices for quantification, and highlighting different methods. It also includes specific … Read more
The International Journal of Medical Informatics recently published the results of a user experience analysis that SIAPS led on e-TB Manager, a digital health tool used to manage TB patients. Using quantitative methods, SIAPS staff analyzed user experiences in nine diverse country health systems that cumulatively bear nearly one-third of the world’s TB burden. The study compared … Read more
TB patients on second-line treatment experience a significant number of adverse effects. Some TB medicines result in adverse effects that, if not continuously monitored, can become serious and/or permanent and may hinder patient adherence to treatment. A number of other risk factors can also impact the safety of patients taking TB medicines, including drug interactions … Read more
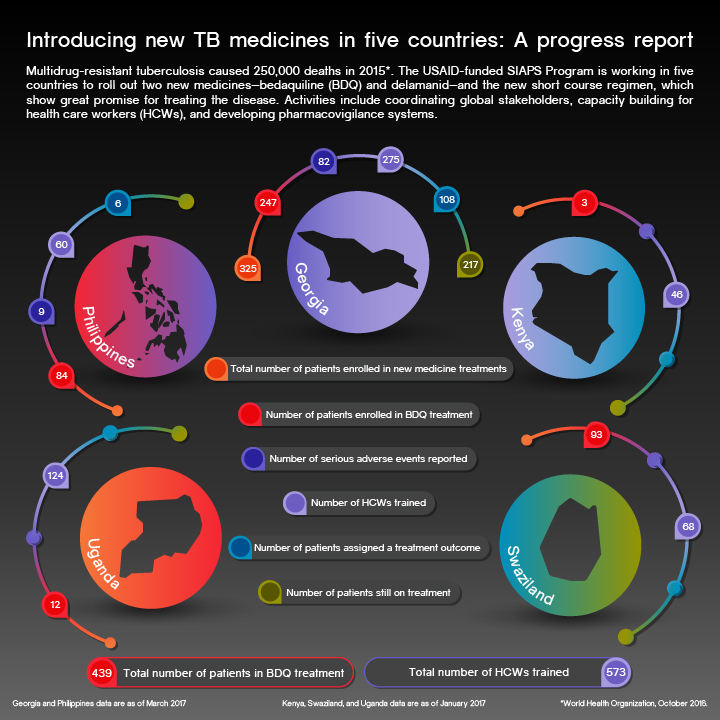
Click on graphic to open larger version.
With the introduction of new anti-tuberculosis (TB) medicines and novel TB treatment regimens in the Philippines, active pharmacovigilance is needed to ensure that both patient safety and the effectiveness of the treatment are monitored. As an important step in pharmacovigilance implementation, the Philippine Department of Health – Pharmaceutical Division (DOH-PD) has adopted the Pharmacovigilance Monitoring … Read more
To begin the project, SIAPS helped coordinate regular planning meetings and discussions with the NTP’s Drugs and Supplies Management (DSM) sub-technical working group. SIAPS then facilitated collaboration among the NTP, the PD, WHO, and the GDF.