
Project dates: September 2011 – September 2016

Project dates: September 2011 – September 2016

An interview with Andwele Mwansasu, MD, SIAPS Senior Technical Advisor What innovations in fighting malaria over the past five years have particular promise? Has there been a watershed moment? With decreasing malaria burden all over the world, a lot has been happening in the malaria world to sustain these gains. Countries are able to detect … Read more
By Claude Bahati, Deputy Country Project Director, SIAPS, Guinea Accurate quantification for malaria programs, which involves forecasting the quantities needed and planning for the procurement of appropriate pharmaceuticals and supplies, is essential to ensuring that patients receive a continuous supply of commodities. The US Agency for International Development (USAID)-funded Systems for Improved Access to Pharmaceuticals … Read more
TB patients on second-line treatment experience a significant number of adverse effects. Some TB medicines result in adverse effects that, if not continuously monitored, can become serious and/or permanent and may hinder patient adherence to treatment. A number of other risk factors can also impact the safety of patients taking TB medicines, including drug interactions … Read more
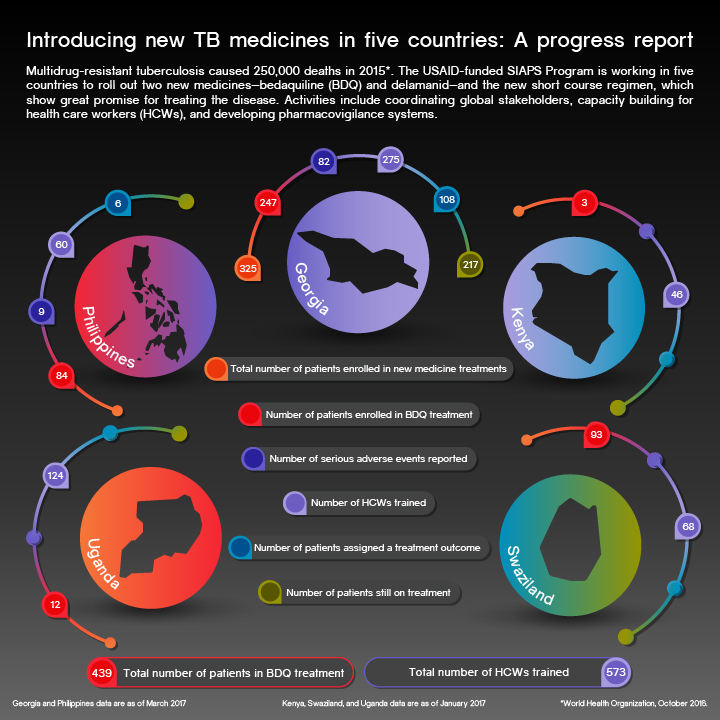
Click on graphic to open larger version.
With the introduction of new anti-tuberculosis (TB) medicines and novel TB treatment regimens in the Philippines, active pharmacovigilance is needed to ensure that both patient safety and the effectiveness of the treatment are monitored. As an important step in pharmacovigilance implementation, the Philippine Department of Health – Pharmaceutical Division (DOH-PD) has adopted the Pharmacovigilance Monitoring … Read more

To begin the project, SIAPS helped coordinate regular planning meetings and discussions with the NTP’s Drugs and Supplies Management (DSM) sub-technical working group. SIAPS then facilitated collaboration among the NTP, the PD, WHO, and the GDF.

SIAPS, in partnership with the FDA, NTP, and LCP-NCPR, conducted a readiness assessment to determine the current information technology (IT) infrastructure, human resources, processes, and data management and quality control mechanisms available and to identify gaps in the current PV recording and reporting of patients in the seven Programmatic Management of Drug-Resistant TB (PMDT) treatment … Read more
Compiled by Wezi Tjaronda (SIAPS), Samson Mwinga (SIAPS), Harriet Kagoya (SIAPS), and Greatjoy Mazibuko (SIAPS) At the Kaisosi settlement in Rundu, Namibia, antiretroviral therapy (ART) patients who were once lost to follow up (LTFU) are returning for care. One of these patients, Domingos Christophine*, a 39-year-old single mother, began ART at the Kuisebmund clinic in … Read more

Pour informer le Ministère de la santé (MS) de la Guinée des besoins du pays et assurer un approvisionnement suffisant en produits antipaludiques, il est essentiel de développer des prévisions précises et reproductibles et un plan d’approvisionnements pour les besoins futurs. A cette fin, le groupe technique – gestion des achats et des stocks (GT-GAS), … Read more