The Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program proposed a methodology for reviewing lists of essential medicines (LEM) and formularies, with the potential to achieve optimum results in short periods of time and with a minimal investment of resources. The methodology consisted of identifying an expert international pharmacologist with experience in the LEM process and the … Read more
The objective of this study was to determine, through secondary sources, the impact of SUGEMI on the operational efficiency of the supply system and the availability of general-use medicines in the primary care units (unidades de atención primaria, or UNAP), where SUGEMI is fully implemented. With these ends, interviews were held with 122 personnel responsible … Read more
La República Dominicana inicio en el año 1997 el proceso de reforma y modernización del sector salud, cuyos principios fundamentales descansan en la calidad, integridad de las acciones de salud, expansión y cobertura universal, separación de funciones para la equidad en el acceso y una mayor eficiencia de los recursos. En el 2005 se presenta la Política Farmacéutica … Read more
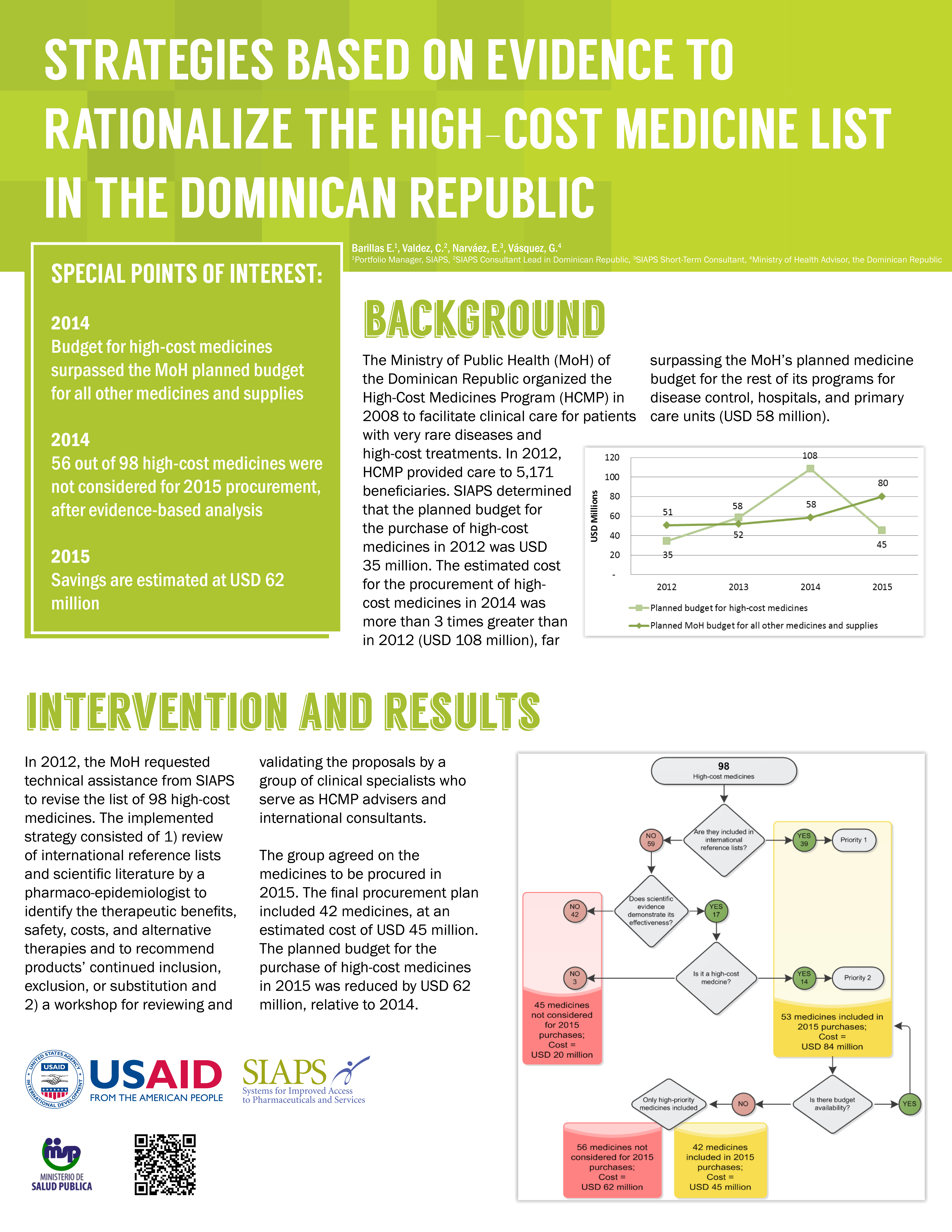
Barillas E, Valdez C, Narvaez E, Vasquez G. Strategies Based on Evidence to Rationalize the High-Cost Medicines List in the Dominican Republic. Poster presented at the ISPOR 18th Annual European Congress in Milan, Italy, November 7-11, 2015.
George A, Valdez C, Herrera M, Barillas E. Building a supply chain approach for an improved laboratory sample referral network in the Dominican Republic. Poster presentation at the People that Deliver Conference, Copenhagen, Denmark. Published in the Journal of Pharmaceutical Policy and Practice. View the abstract
Barillas E, Valdez C, Diaz P, Tapia ME. Combination of on- and off-site training contributes to strengthening the unified pharmaceutical system in the Dominican Republic. Oral presentation at the People that Deliver Conference, Copenhagen, Denmark. Published in the Journal of Pharmaceutical Policy and Practice. View the abstract
Ghoneim R, Nfor E, Tukai M, Barillas E, Saleeb S, Valdez C (2014, November). Adoption of the integrated management information system for pharmaceuticals and medical supplies (SUGEMI) to optimize ARV supply in the Dominican Republic. Presentation at the 7th Global Health Supply Chain Summit. Copenhagen, Denmark.
C Valdez, Conference, Dominican Republic, E Barillas, E Nfor, HIV/AIDS, M Tukai, Pharmaceutical Management Information System, Presentation, R Ghoneim, S Saleeb, SUGEMI
Download English The implementation of an integrated pharmaceutical system in the Dominican Republic is correcting critical problems in medicine supply and, as an unexpected effect, is contributing to the completion of the health reform process and to the implementation of public administration decentralization and transparency. Download Spanish La implementación de un sistema integrado de medicamentos … Read more