As a part of its ongoing support to build the capacity of the DGDA, the Systems for Improved Access to Pharmaceuticals and Services (SIAPS) team conducted a rapid assessment of the capacity of the Good Manufacturing Practices (GMP) inspection program at the Directorate General of Drug Administration (DGDA). GMP is part of the quality assurance … Read more
Assessment, Bangladesh, Directorate General of Drug Administration, E Kim, Good Manufacturing Practices (GMP), governance, Inspection, J Aimiuwu, M Thumm, MH Anisfeld, Quality Assurance, regulations, SOPs
The purpose of this standard operating procedure is to outline a step-by-step approach for undertaking active drug safety monitoring for the 9-month MDR-TB treatment regimen and other novel medications and regimens for TB and MDR-TB.
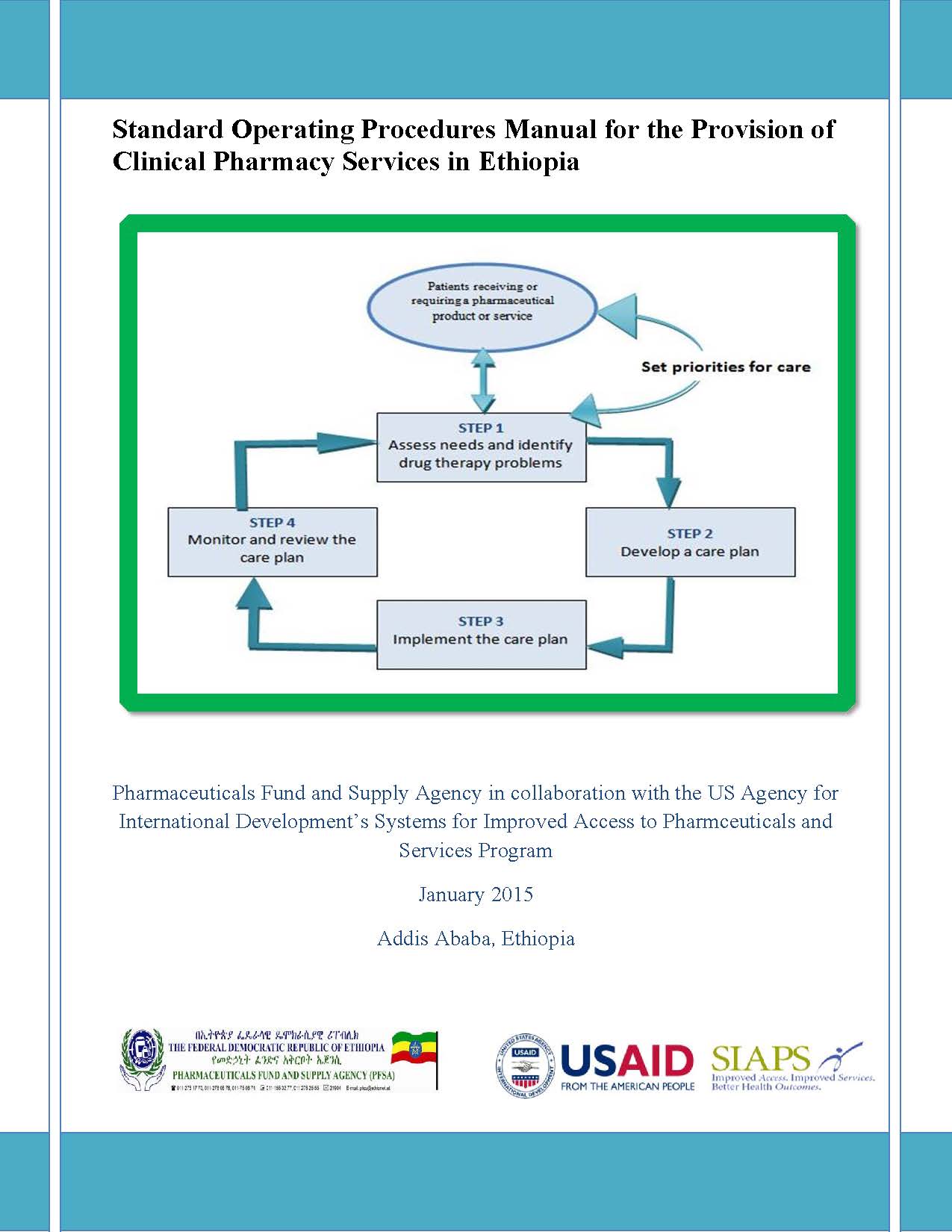
The direct involvement of pharmacists in patient care (clinical pharmacy services) is a key intervention to optimize the outcomes of medicine therapy, thereby improving the quality of patient care. The Pharmaceuticals Fund and Supply Agency (PFSA) has been collaborating in efforts to implement clinical pharmacy services in the Ethiopian health care system. As part of … Read more