By Francis Aboagye-Nyame, SIAPS program director Greatjoy Mazibuko was a pharmacist at the Oshakati Intermediate Hospital in Namibia working with ART patients. Every day, he rose at dawn, not knowing how many patients he would have that day. He often worked for 12 hours or more; patients were kept waiting for hours, perhaps having traveled … Read more
Archive Blog
Case studies in strengthening pharma systems: Evidence of lasting progress
By Francis Aboagye-Nyame, SIAPS Project Director Over its six years working in dozens of countries, SIAPS has carried out a vision for health system strengthening that USAID developed and has supported for more than two decades. In partnership with countries and organizations, the agency has led pharmaceutical systems strengthening interventions that have helped countries … Read more
Setting the stage for pharmaceutical system reform in Ukraine
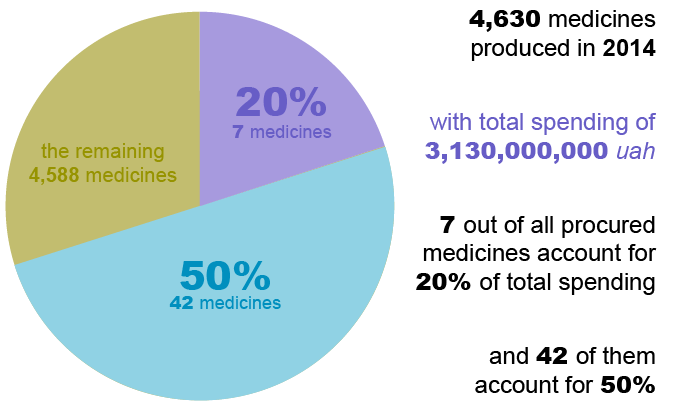
By Juanita Folmsbee, Ukraine Country Project Director, SIAPS and SAFEMed To be fully effective, health system strengthening projects should have sustainable impact and lay the groundwork for future progress. Here’s how SIAPS’ work supported health system reform in Ukraine. SIAPS worked in Ukraine for four years, from 2013 through 2017. Ukraine has the most severe … Read more
Strong pharmaceutical systems run on sound data
By David Mabirizi, SIAPS Deputy Director, Country Programs Q. What does managing pharmaceutical services have in common with flying planes or performing open-heart surgery? A. All of these are complex operations that need accurate, timely information to make effective, life-saving decisions. Here’s why: Sound data helps pharmaceutical managers ensure an uninterrupted supply of medicines; quantify … Read more
Special preview: A new tool to measure pharmaceutical systems strengthening
By Helena Walkowiak and Maura Soucy Brown In SIAPS’ upcoming webinar on defining and measuring pharmaceutical systems strengthening (PSS), we’ll talk briefly about the development of a new tool that SIAPS and its partners are piloting. It’s intended to help the field measure the impact of PSS efforts. Some background on this activity: SIAPS has … Read more
SIAPS Voices Q&A: No time to waste in preventing AMR
As part of World Antibiotic Awareness Week 2017, we present a Q&A with Mohan P. Joshi, MBBS, MSc, MD, SIAPS principal technical advisor. Dr. Joshi is responsible for providing technical guidance and support in the planning and implementation of rational medicine use and antimicrobial resistance (AMR)-related activities. Are there new AMR threats that are particularly worrying? … Read more
SIAPS Voices: Working for improved access to HIV treatment in Namibia
A Q&A with Greatjoy Mazibuko, SIAPS Senior Technical Manager in Namibia You’re working in Namibia, which has one of the highest prevalence rates of HIV in the world. How does this affect life in the country? The prevalence of HIV infection stood at 17.2% in 2016, which is relatively high for sub-Saharan Africa, but it’s … Read more
A Rebirth of Sierra Leone’s Pharmaceutical System
When the World Health Organization declared the Ebola epidemic over on November 7, 2015, about 40% of the 8,704 people infected had died. The country’s health system, or what was left of it after a decade of civil war that ended in 2002, was ailing, too. Some health workers had become ill or left, leaving … Read more
Rebuilding Sierra Leone’s pharmaceutical system post-Ebola
An interview with Murtada Sesay, country project director in Sierra Leone. SIAPS is working on a two-year project funded by USAID to help rebuild and strengthen the country’s pharmaceutical system following the Ebola epidemic. What’s SIAPS doing in Sierra Leone? Our systems strengthening project there was a consequence, not only of the more recent Ebola, … Read more
What’s the ROI on investing in health systems?
By Francis (Kofi) Aboagye-Nyame, SIAPS Director You can’t put a price tag on health, but we do have budgets that determine what we can spend to help countries improve theirs. At SIAPS, that means we need to look closely at the impact each dollar we spend has on achieving our goal: strengthening the management of … Read more


