STGs are designed to assist health care professionals in making decisions about appropriate, effective patient care. However, health managers often have trouble setting and meeting the high standards required of modern, developed health care systems. With stakeholders expressing concern over issues such as strength of evidence, transparency, conflicts of interest, and effective implementation, it is … Read more
Through its support of governments in low- and middle-income (LMIC) countries across the globe, SIAPS Program has helped many governments address the data management challenges facing their health systems. Governments and their partners are acutely aware of the need for innovative ways to reduce the data burden on health workers. To define and quantify the … Read more
In many countries, weaknesses in information systems for TB logistics management have resulted in a lack of quality data for decision making. In addition, challenges related to the management of second-line drugs are emerging and may contribute to stock-outs. Those challenges include (a) the lengthy duration of drug-resistant TB treatment, (b) the short shelf life … Read more
La présente étude vise à examiner la faisabilité d’une introduction des tests de diagnostic rapide (TDR) et combinaisons thérapeutiques à base d’artémisinine (CTA) subventionnés pour la prise en charge du paludisme dans les pharmacies du secteur privé au Mali, et déterminer quels éléments doivent être pris en compte afin de garantir que cette introduction s’effectue … Read more
A Yattara, C Touré, D Kone, E Rutta, governance, M Konate, Malaria., Mali, S Diarra, S Doumbia, Technical Report
These guidelines provide instructions for applicants preparing a Common Technical Document (CTD) for the registration of medicines for submission to the Directorate General of Drug Administration (DGDA). The document describes how to organize applications based on the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines on the … Read more
This report presents highlights of SIAPS’ activities organized both by intermediate result area, representing multiple countries where we work, as well as by our global, regional, and country portfolios for the April through June 2015 period.
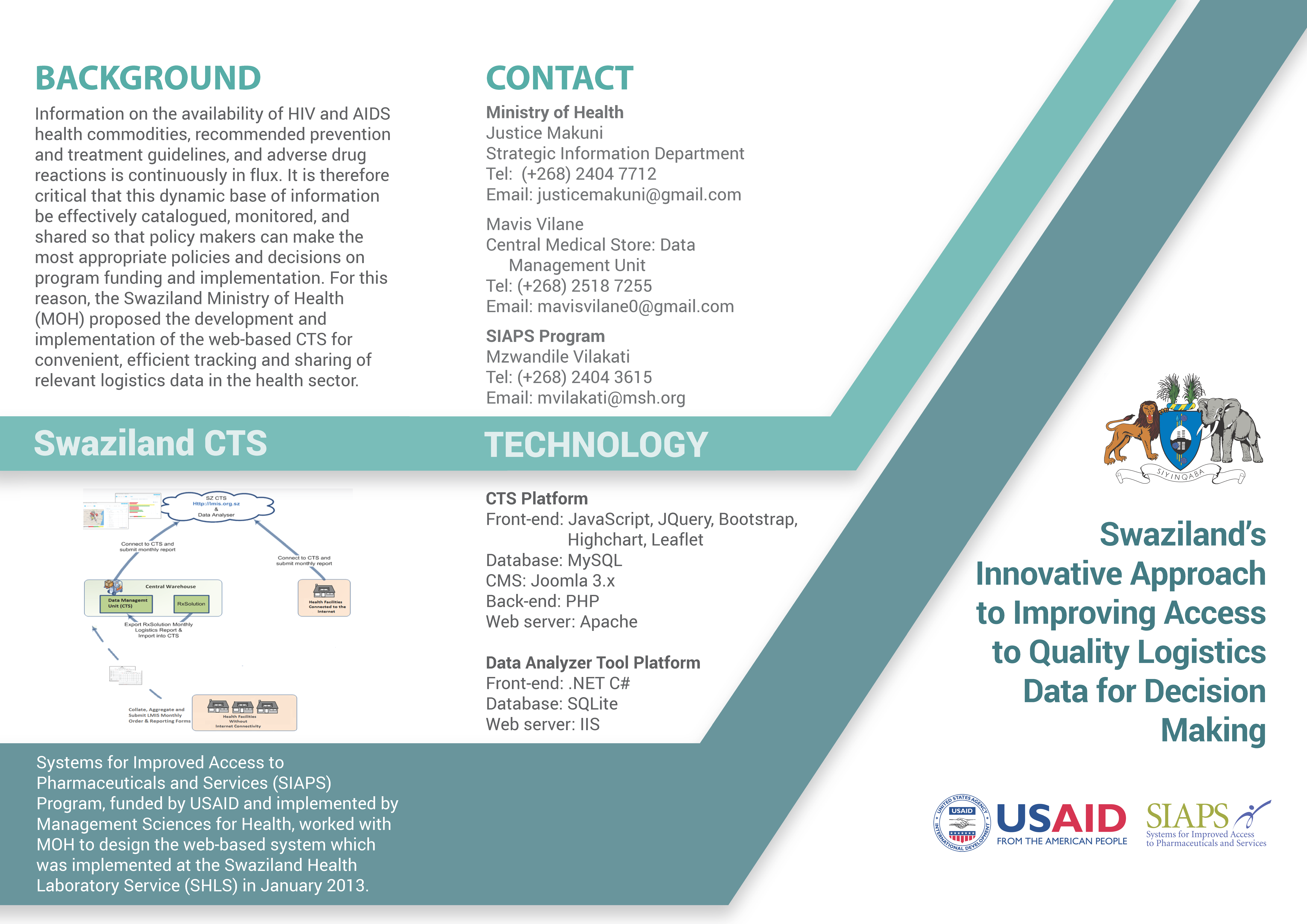
Swaziland Ministry of Health (MOH) proposed the development and implementation of the web-based CTS for convenient, efficient tracking and sharing of relevant logistics data in the health sector. SIAPS worked with MOH to design the web-based system which was implemented at the Swaziland Health Laboratory Service (SHLS) in January 2013.
The overall goal of the SIAPS/Bangladesh program is to build the capacity of MOHFW and its key directorates—DGFP, DGHS, DGDA, and HED—and other indigenous institutions to efficiently and effectively manage their procurement and supply chain management activities. Special focus will be given to TB commodity management at all levels. Read about their progress toward this … Read more
The entire Nigerien population is exposed to malaria. However, pregnant women and children under 5 years of age are the most vulnerable groups and frequently develop severe malaria. The epidemiology of malaria in Niger is characterized by stable endemicity with a seasonal increase during and after the rainy season (June to December). The latest outbreak … Read more
Management Sciences for Health (MSH) identified the Accreditation Council for Pharmacy Education (ACPE) as one of the partners in the Systems for Improved Access to Pharmaceuticals and Services (SIAPS) program proposal funded by the US Agency for International Development (USAID). ACPE is the national accreditation agency for quality assurance of pharmacy education in the United … Read more