Although SIAPS introduced a number of automated inventory control tools, such as the Facility Electronic Stock Card (FESC) and Electronic Dispensing Tool (EDT) for district hospitals, since June 2015, at the primary health care (PHC) level, inventory control and storage practice are a major challenge and the major cause of stock-out of medicines for antiretroviral … Read more
In an effort to improve the health status of the Beninese population, a priority activity included in the 2015 convention between the US Government, represented by USAID, and the Benin Government, represented by the Ministry of Health (MOH), was to conduct a comprehensive assessment of the public health supply chain, focused on essential medicines that … Read more
A Gbaguidi, Benin, E Nfor, Human Resource Management, L Maxim, M Levenger, Pharmacovigilance, Procurement, Quantification, S Conesa, Supply chain management, Technical Report
This guide is written for the DOH Philippines’ LMD. It can be used as support material in the training and development of new and existing staff involved in warehouse and distribution operations at all levels, particularly those who are involved in the process of receiving, putaway and storing, picking and packing, and dispatching of FP, … Read more
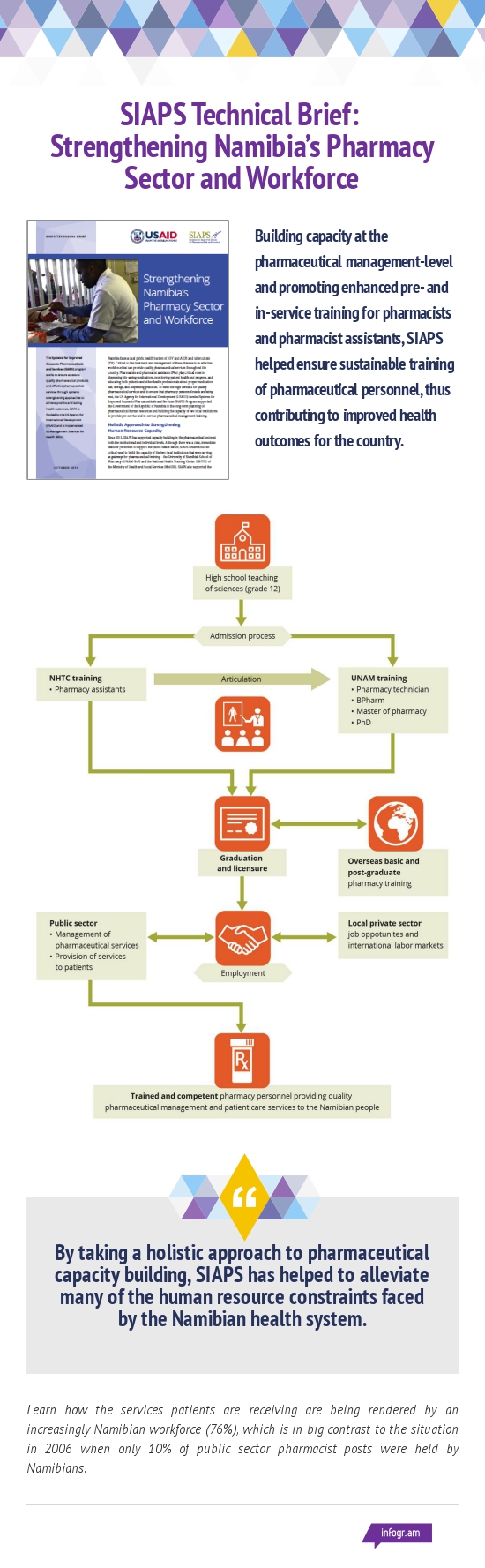
SIAPS supported the Ministry of Health and Social Services to enhance capacity of pharmaceutical HR to manage pharmaceutical services in public health facilities in Namibia. The major objectives of the activities were to build HR capacity in pharmaceutical management and service delivery for improved HIV and AIDS treatment outcomes and improve availability and use of … Read more
According to the World Health Organization, many countries spend 30–40% of their health care budgets on medicines and medical commodities, and a significant amount of the funds are wasted because of irrational medicines use and inefficiencies in stock management due to lack of skills. Other serious problems that health care organizations face include the overuse … Read more
The purpose of this activity was to design and implement warehouse operations system improvements for CECOMA, and to design and implement a human resource capability development and performance improvement (HRCD&PI) program based on the identified gaps. The assignment entailed conducting a rapid analysis of warehouse and distribution system capacity, including staff capacity, to identify gaps … Read more
In Namibia, USAID has been providing funding for technical assistance in the areas of pharmaceutical management and systems strengthening since 2003. During this period, the Rational Pharmaceutical Management Plus (RPM Plus) and Strengthening Pharmaceutical Systems (SPS) programs were implemented. RPM Plus supported interventions that largely focused on strengthening systems for the antiretroviral therapy (ART) and … Read more
To assess the feasibility of launching a pharmacy training program or programs in Swaziland, the assessment considered the establishment of different pharmacy training programs with different training models, taking into consideration market requirements in both the public and private sectors. The feasibility assessment recommended the ideal curriculum to be adopted for Swaziland.
Namibia faces a dual public health burden of HIV and AIDS and tuberculosis (TB). Critical to the treatment and management of these diseases is an effective workforce that can provide quality pharmaceutical services throughout the country. Pharmacists and pharmacist assistants (PAs) play critical roles in dispensing life-saving medications, monitoring patient health and progress, and educating both patients and other health … Read more
With the shift from a disease landscape that focuses on the treatment of acute and short-term illnesses to one that faces an increasing burden of chronic diseases that may require life-long medicine use, the role of medicines in ensuring a healthy population is more important than ever. However,even when medicines are available, patients may not … Read more
A Clark, Adherence, Antimicrobial Resistance, Governence, guidance document, Human Resource Management, M Ludman, MP Joshi, pharmaceutical financing, pharmaceutical services, SIAPS