Click on graphic to open larger version.

Click on graphic to open larger version.
In April 2015, the US Agency for International Development (USAID) and Janssen Therapeutics officially launched the bedaquiline donation initiative. As part of this initiative, Janssen committed to providing bedaquiline at no cost to 30,000 patients with multidrug-resistant tuberculosis (MDR-TB) over a four-year period. Bedaquiline is the first anti-TB medicine to be approved by the U.S. Food … Read more
In 2012, bedaquiline was conditionally approved by the U.S. Food and Drug Administration for the treatment of drug-resistant tuberculosis (TB), making it the first new TB drug to enter the market in more than 40 years. With the rise of multidrug-resistant and extensively drug-resistant TB leaving patients with fewer treatment options, the approval of bedaquiline … Read more
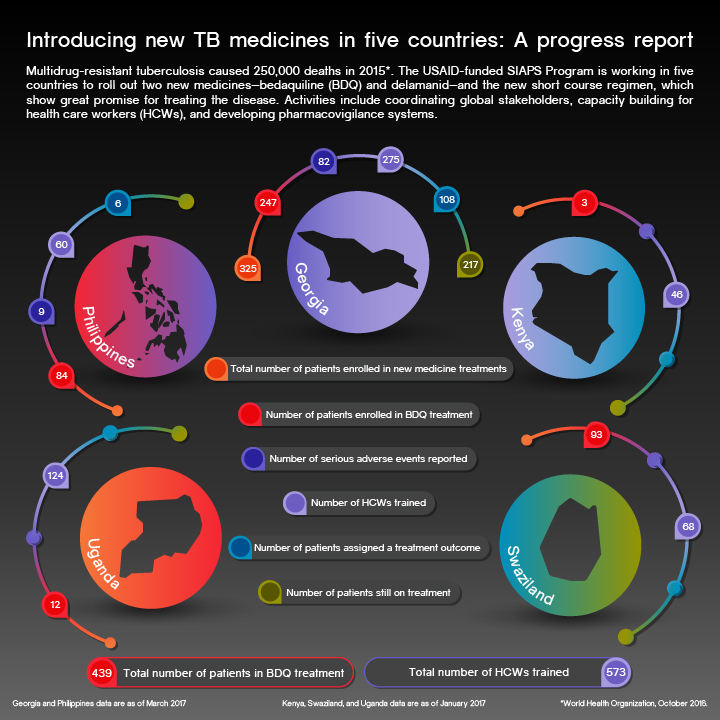
In April 2015, USAID and Janssen Therapeutics officially launched the bedaquiline (BDQ) donation initiative, under which Janssen committed to providing free BDQ to 30,000 patients with multidrug-resistant tuberculosis (MDR-TB) over a four-year period. Along with delamanid, BDQ is one of only two new TB medicines released to the market in over 40 years. These medicines … Read more
In April 2015, USAID and Janssen Therapeutics officially launched the bedaquiline (BDQ) donation initiative, under which Janssen committed to providing free BDQ to 30,000 patients with multidrug-resistant tuberculosis (MDR-TB) over a four-year period. Along with delamanid, BDQ is one of only two new TB medicines released to the market in over 40 years. These medicines … Read more
In April 2015, USAID and Janssen Therapeutics officially launched the bedaquiline donation initiative, under which Janssen committed to providing free bedaquiline to 30,000 patients with multidrug-resistant tuberculosis (MDR-TB) over a four-year period. Bedaquiline is the first anti-TB medicine to be approved by the U.S. Food and Drug Administration in more than 40 years, and is … Read more
In April 2015, USAID and Janssen Therapeutics officially launched the bedaquiline donation initiative, under which Janssen committed to providing free bedaquiline to 30,000 patients with multidrug-resistant tuberculosis (MDR-TB) over a four-year period. Bedaquiline is the first anti-TB medicine to be approved by the U.S. Food and Drug Administration in more than 40 years, and is … Read more