SIAPS mobilized stakeholders from the Swaziland National AIDS Program (SNAP) and the National Tuberculosis Control Program (NTCP) to introduce and implement the Sentinel Site-based Active Surveillance System for Antiretroviral and Anti-TB (SSASSA) treatment programs. SIAPS partnered with the Pharmacovigilance Unit of the MOH to create the protocol and tools for the electronic SSASSA system, and developed a patient recruitment system at HIV and TB … Read more
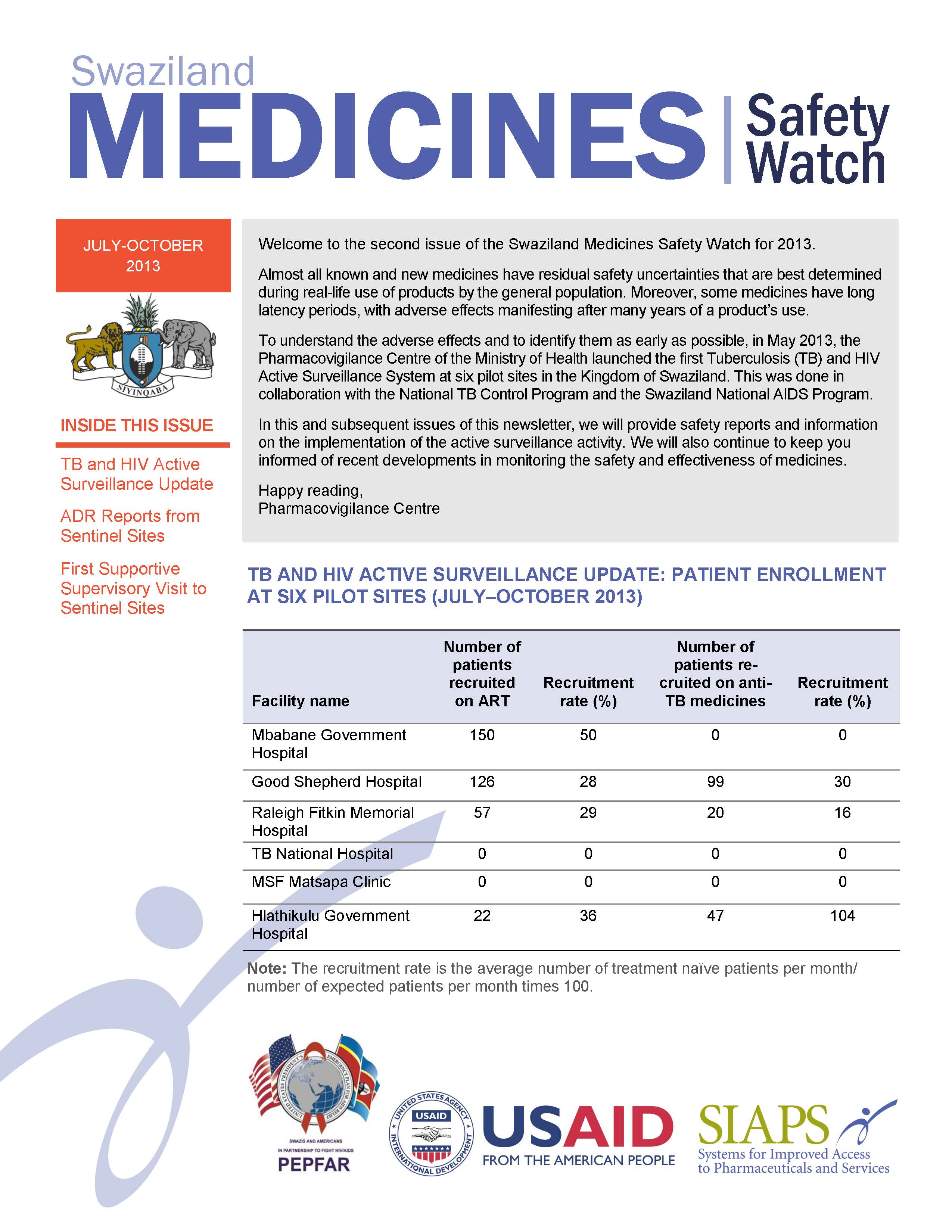
In collaboration with the National TB Control Program and the National AIDS Program, Swaziland’s Pharmacovigilance Centre launched the first Tuberculosis (TB) and HIV Active Surveillance System at six pilot sites in May 2013. The Swaziland Medicines Safety Watch provides safety reports and information on the implementation of the active surveillance activity.
Adverse drug events among patients beginning new medicines can have disastrous consequences for treatment outcomes. The Swaziland Ministry of Health’s (MoH) National Pharmacovigilance Centre, the National Tuberculosis Control Programme, and the Swaziland National AIDS partnered with the US Agency for International Development (USAID)-funded Systems for Improved Access to Pharmaceuticals and Services (SIAPS) to launch an … Read more