Project dates: September 2011–December 2016
Background
South Africa, with a population of approximately 50 million, has one of the highest HIV disease burdens in the world. In 2009, an estimated 5.7 million people in South Africa (17% of the population) were living with HIV. South Africa’s HIV epidemic is defined as hyper-endemic[1], with an HIV prevalence of 16.9% among 15 to 49 year olds and 29.3% among antenatal women[2].
The high burden of HIV in South Africa has a direct impact on the burden of TB and other co-infections. The World Health Organization estimated 490,000 TB cases in South Africa, 60% of which were co-infected with HIV[3]. In 2009, 114,500 TB patients (up from 90,000 in 2008) tested positive for HIV and 48,300 patients (up from 22,100 in 2008) were living with HIV and receiving antiretroviral therapy (ART). South Africa has the second highest global TB incidence (971 cases/100,000 population). Multidrug-resistant and extensively drug-resistant TB are on the rise.
In 2011, SIAPS with funding from PEPFAR/USAID began providing technical assistance to the South African Government to strengthen the pharmaceutical system.
Selected Activities
SIAPS South Africa’s objectives were to:
Results
In 2014, the NDOH endorsed RxSolution as a tool to manage pharmaceutical inventory. RxSolution is interoperable and interfaces with five other information systems—Patient Administration and Billing, Delta 9, Medsas, Tshwane Biometric System, and RxPMPU (Provincial Medicine Procurement Unit).

-Strengthened patient-centered pharmacovigilance in the North West and Mpumalanga provinces.
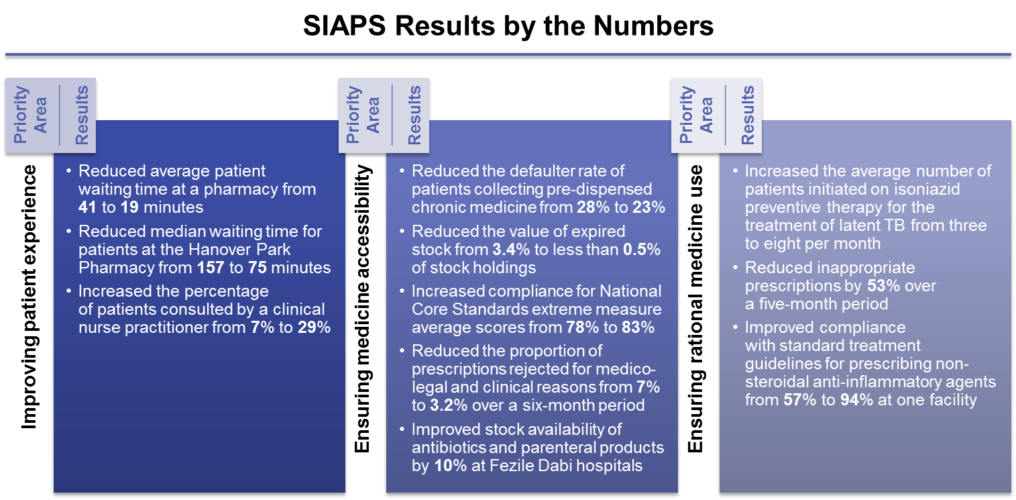
-SIAPS actively supported the establishment and strengthening of PTCs at the provincial, district, and facility levels. By promoting rational medicine use, the Eastern Cape, Gauteng, and Limpopo provinces have projected a total combined annual savings of R 24 million (approximately USD 1.77 million) per 100,000 patients using selected medicines for chronic diseases.
Project Legacy
Interventions provided an immediate response to urgent pharmaceutical problems, developed the policy framework, and built human capacity to strengthen the health system. SIAPS played a crucial role in enhancing the coordination and management of pharmaceuticals at the national and facility levels. Users throughout the health system have increased access to information for decision making. These interventions contributed to the increased capacity of MOH personnel and raised awareness of the need to improve governance and information systems in the pharmaceutical sector.
Resources
- South Africa Pharmaceutical Leadership Development Program
- Infection control assessment tool: a standardized approach for improving hospital infection control practices
- Improving infection prevention and control practices at health facilities in resource-limited settings: SIAPS technical report
- Antiretroviral cohort adverse event monitoring in Kwazulu-Natal
- Using electronic pharmacy dispensing data for surveillance of outpatient antibiotic consumption and monitoring of antibiotic prescribing practices at district and provincial hospitals in the south African public sector
- Guidelines for implementation of pharmaceutical and therapeutics committees in Gauteng Province
- Using a Health Systems Strengthening Approach to Contain AMR: a South African Experience
- Other documents
———————————————————————————————————————
[1] According to UNAIDS (2008), hyper-endemic scenarios refer to those areas where HIV prevalence exceeds 15% of the population and are driven by extensive heterosexual multiple concurrent partner relations with low and inconsistent condom use.
[2] Republic of South Africa, Country Progress Report on the Declaration of Commitment on HIV/AIDS, 2010 Report (March 2010).
[3] World TB Report, 2010.