Project Dates: 2011 – 2018
Background
The SIAPS program in Bangladesh worked to address barriers to access to essential health commodities. The objective was to strengthen the ability of policy makers, health care providers, and institutions to improve commodity management and ensure the continuous availability of commodities. SIAPS focused on improving governance, procurement, and the timely availability of reliable data to support evidence-based decision making. In collaboration with the Government of Bangladesh and other stakeholders, SIAPS worked to build sustainable capacity of the Ministry of Health and Family Welfare (MoHFW) and other institutions to carry out pharmaceutical management functions that would improve health service delivery in the country and achieve health outcomes. SIAPS supported the MoHFW’s Directorate of Family Planning, Directorate General Drug Administration (DGDA); Directorate of Health Services (DGHS), including the Central Medical Stores Department; the National TB Program (NTP); and the maternal, newborn, and child health (MNCH) program in implementing system strengthening activities.
Project Highlights
Technical assistance was provided to the Directorate General Family Planning (DGFP) and DGHS to successfully introduce the Procurement and Logistic Management Cell (PLMC) in 2012. In coordination with the PLMC, SIAPS:
- Created a standard table of equipment for optimal medical equipment for primary- and secondary-level health facilities and supported needs-based procurement planning and budgeting
- Developed a framework agreement, which is a standard bidding document that allows for preauthorization of bidders and a more efficient multiyear procurement with flexible delivery schedules
- Built the human resource capacity of 11,858 health workers to manage pharmaceuticals
- Established and scaled up the Upazila Inventory Management System (UIMS), procurement tracker, and Supply Chain Management Portal (SCMP), which the DGHS has implemented in 20 districts
- Established an electronic logistic management information system (eLMIS) dashboard for 25 priority MNCH medicines in 19 districts using the DHIS2 platforms under the DGHS
- Developed an asset management system that was field tested in the Moulvi Bazar district
The MoHFW/NTP implemented QuanTB and established a Procurement and Supply Management (PSM) unit to strengthen pharmaceutical management. The PSM unit in the NTP facilitates regular supply planning to minimize the risk of overstocks or stock-outs and has adopted an early warning system.
The DGDA made significant progress in improving pharmaceutical sector governance and strengthening regulatory systems. The Adverse Drug Reaction Monitoring Cell was upgraded to serve as the National Drug Monitoring Centre for Bangladesh and was awarded full membership in WHO’s Monitoring Centre.
The MoHFW developed a standardized list of equipment required at various levels of the health care system and a pricing guide, and equipment status is tracked through the portal.
The NTP has implemented e-TB Manager, a case management system, at 210 DOTS sites, including 4 MDR-TB treatment sites. Standard operating procedures for TB drug supply management were updated. SIAPS conducted quantification training for NTP staff and partner organizations. It also reviewed the quantification figures for country submission to the Global Fund New Funding Model and, based on SIAPS recommendations and support, the NTP revised and published the TB pharmaceutical guidelines and reporting forms.
Results
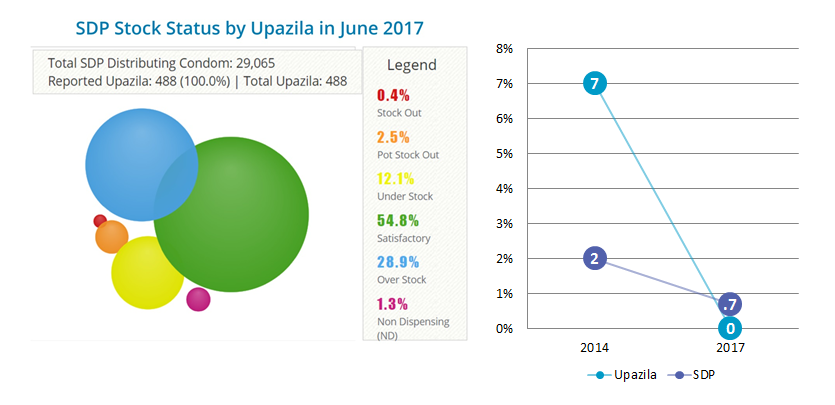
The MoHFW has eliminated stock-outs of five reproductive health tracer commodities at the national level. All 32 line directors now develop their procurement plans using the SCMP.
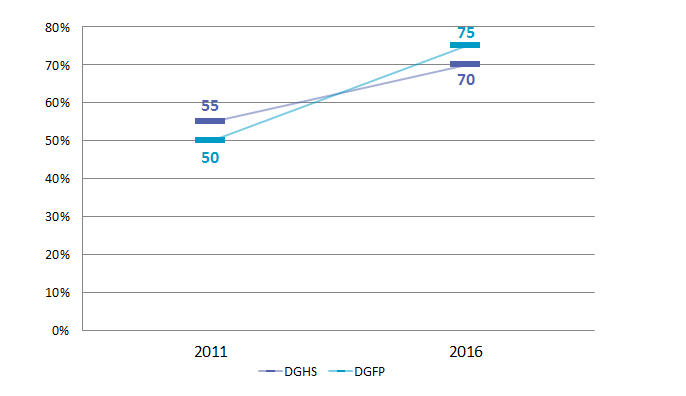
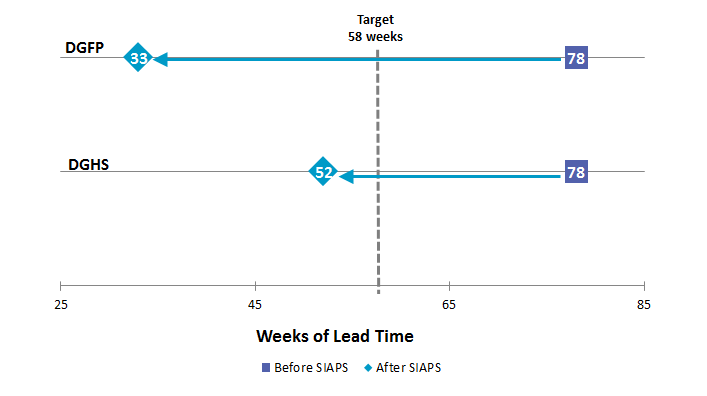
The length of the procurement process decreased by 57.7% (from 78 to 33 weeks) at the DGFP and by 33.3% (from 78 to 52 weeks) at the DGHS. Procurement efficiency has improved, and the DGFP and DGHS procurement packages that are on schedule have increased from 50% and 55% in 2011 to 75% and 70% in 2016, respectively.
Postponement and reallocation of second-line TB medicines shipments minimized overstocking. As a result, Bangladesh saved more than USD 899,000 of country and donor funding from potential wastage. As of 2015, The MoHFW had saved USD 6.38 million from improved quantification of family planning/reproductive health, maternal and child health, and TB commodities.
Project Legacy
The established DGDA system sets a strong foundation for evaluating and registering medicines that meet international standards. Common Technical Documents and standard operating procedures developed through the taskforce will further standardize the medicine registration process and adaptation of the online regulatory management system (Pharmadex).
The PLMC now serves as the coordinating body to monitor and supervise procurement and supply chain functions. The tools developed and implemented by SIAPS are being effectively used and provide real time data for decision making. Enhanced availability and visibility of commodity information for managers has enhanced the use of evidence in decision making. Through the use of the SCMP and the establishment of the PMLC within the MoHFW to manage the portal, stock-outs have decreased significantly and there is uninterrupted availability of commodities at all levels of the health system.
Resources
- Sustainability Plan for SCMP
- WHO-UMC Welcomes Bangladesh as 120th Full-Member Country
- National Reproductive Health Commodities Forecasting Bangladesh 2017–2021
- Strengthening Governance in Procurement in Bangladesh
- DHIS2 and e-TB Manager Interoperability: Creating a Stronger Digital Health System in Bangladesh
- Saving Lives of Women and Children
- Celebrating the Success of SIAPS in Bangladesh
- Other Documents