Background
Togo, a country in West Africa on the Gulf of Guinea, had an estimated 2016 population of 7.6 million.[1] In 2016, the country reported 4,100 new HIV infections and 5,100 AIDS-related deaths, although those numbers have been steadily declining since 2010.[2]
In 2012 and 2013, alerts of stock-outs of ARVs emerged in a number of countries in West Africa, including Togo. Among the root causes were a lack of accurate information on HIV and AIDS patients and commodity information for faster decision making. The notifications were always last minute, creating emergencies that could have otherwise been avoided.
Project Highlights
SIAPS supported the National AIDS Control Program (PNLS) to conduct a baseline situation analysis that informed the development of strategies to enable the country to overcome a persistent lack of information on commodities and predict/avert stock-outs of lifesaving ARVs. Supportive supervision activities were implemented at five pilot sites using the Electronic Dispensing Tool (EDT) to build the capacity of EDT users and identify issues affecting the use of the EDT before a nationwide roll-out. With funding from the Global Fund, the EDT was ultimately rolled out to the entire country.
SIAPS also developed and installed OSPSIDA, a dashboard for HIV commodities in Togo, to enable the PNLS to detect and prevent stock-outs of ARVs. Using OSPSIDA to analyze commodity management and access consumption and shipment data allowed for a thorough analysis of the pipeline of ARVs to determine the risk of a stock-out and when it might occur. For example, a review of OSPSIDA reports highlighted impending stock-outs of two key products—tenefovir-lamivudine-efavirenz and abacavir-lamivudine—if orders were delivered as initially scheduled.
The expected outcomes of these activities were:
- Improved use of the EDT in the management of HIV and AIDS patients in Togo
- Motivation and justification for other West African countries in the project to consider using electronic dispensing tools to improve antiretroviral therapy (ART) information accuracy, availability, and utilization
Results
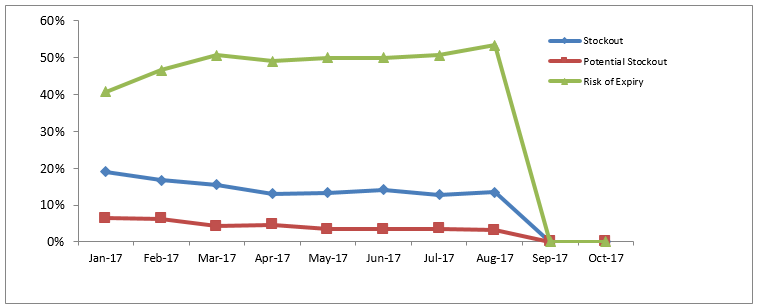
The dashboard allows the country to assess stock status at all levels, which was not possible before OSPSIDA. The consumption data in Togo are integrated across peripheral- and national-level data, providing a more accurate status of stock of commodities available for patient care nationwide and projecting the risk of stock-outs.
There was a significant improvement in the quality of data by using OSPSIDA. The accuracy of closing and opening balances of stock in logistics and management information systems reports increased from 55% to 100% within a year of the deployment of the dashboard, and there was overall improvement in reporting rates and report completeness.
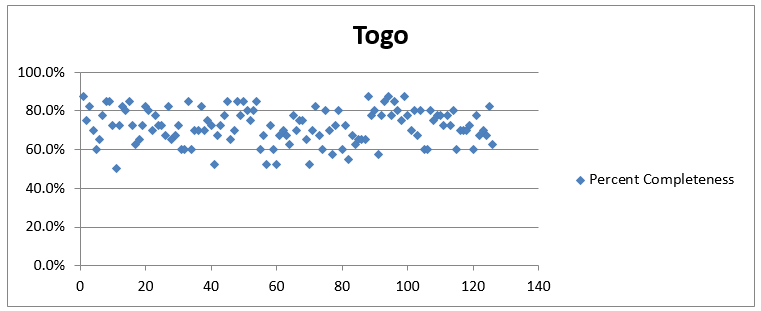
Results from EDT supervisory visits showed improved data quality through key indicators, with 100% concordance between EDT records and prescriptions at each dispensing site. By the end of the project, at least four pilot sites had already abandoned the paper-based dispensing register. In November 2014 and early 2017, OSPIDA reports were used to identify the risk of stock-outs and prevent treatment interruption for the majority of patients. Togo’s stock of ARVs was successfully used to avert a stock-out in Niger as a result of regional collaboration and access to updated stock data.
Deploying OSPSIDA in Togo has significantly enhanced the visibility of supply chain data for timely and evidence-based decisions, thereby contributing to the increased availability of HIV/AIDS products.
In 2014, an estimated 71% of ARVs in Togo were at high risk of stock-outs at the national level, putting 96% of patients at high risk of treatment interruption. The risk of stock-outs decreased to less than 1% by November 2015 with support from SIAPS.
Project Legacy
OSPSIDA will not only provide an early warning of a stock-out risk but also improve data quality management to improve the general management of patient and commodity data for decision making. OSPSIDA will facilitate the understanding of funding and commodity gaps so that stakeholders are informed in time to ensure that resources are available before a stock-out happens.
The achievements in Togo are a clear motivation to the West Africa region, and the prudent use of a dashboard like OSPSIDA can result in enhanced visibility of patient and commodity data, thereby hastening evidence-based decision making and preventing unnecessary stock-outs of life saving medicines.
Resources
- The West Africa Regional Project Dashboard: Better information for Better Decision Making
- Deployment of the HIV and AIDS Commodity Management Tool OSPSIDA in Six Focus Countries in West and Central Africa: Benin, Burkina Faso, Cameroon, Guinea, Niger, and Togo
- HIV and AIDS Commodity Management Tool
- A Protocol for Rapid Situational Analysis of the HIV and AIDS Commodity Supply Chain in Seven Target West African Countries
- Information Systems and Coordination for Managing HIV and AIDS Related Commodities

