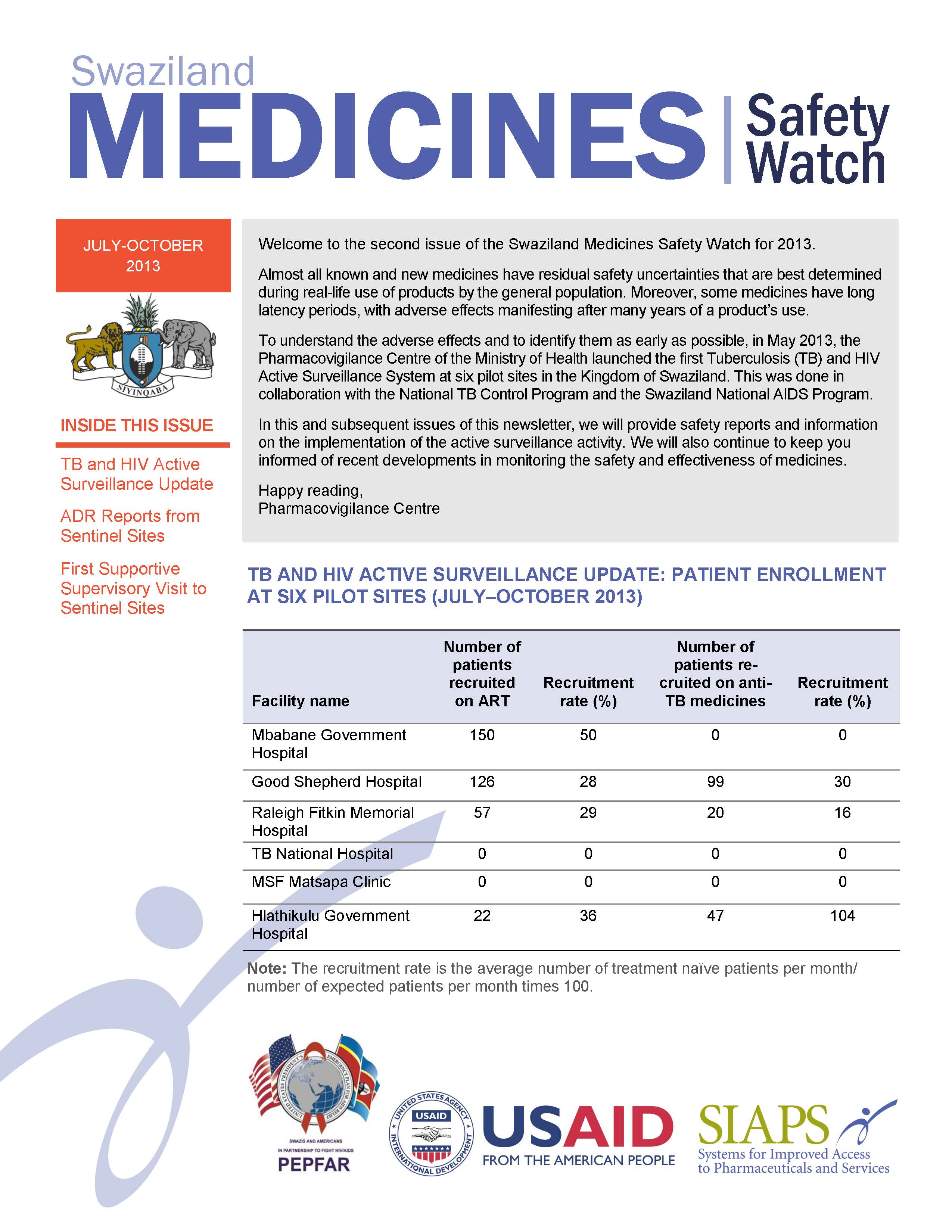
In collaboration with the National TB Control Program and the National AIDS Program, Swaziland’s Pharmacovigilance Centre launched the first Tuberculosis (TB) and HIV Active Surveillance System at six pilot sites in May 2013. The Swaziland Medicines Safety Watch provides safety reports and information on the implementation of the active surveillance activity.


