Treatment guidelines are important to implement safe and effective practices that are based on clinical evidence. The Ethiopian Antiretroviral Therapy (ART) Guidelines, last updated in 2008, were based on the latest WHO recommendations at the time. According to the guidelines, all new patients receiving ART should be on a three-drug regimen that contains the HIV and AIDS medicine tenofovir (TDF), instead of stavudine (D4T). Due to the relative toxicity of D4T, its use was reserved to patients with special needs. The long-term use of D4T can prove harmful, leading to, for example, abnormal distribution of fat on patients’ faces, which could lead to social stigma. The fear of stigma not only reduces the quality of life of patients, but also leads to failure in adhering to treatment.
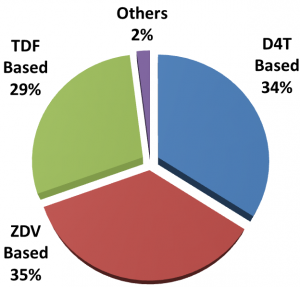
The USAID-funded SIAPS Program routinely monitors ART patient-uptake and drug treatment regimen data from more than 800 ART pharmacies throughout Ethiopia with the help of electronic and manual dispensing tools. Four years into the implementation of the current ART guidelines, analysis of data by SIAPS showed that 34% of patients were on a drug regimen containing D4T. This information was communicated to USAID, whereupon it took the initiative, together with the Center for Disease Control, to coordinate a meeting of stakeholders to change the practice that was unnecessarily exposing ART patients to a high risk of toxicity.
 A taskforce was formed under the leadership of the Ethiopian Federal Ministry of Health and it produced supplementary guidelines for managing the transfer of patients currently on stavudine to non-stavudine-containing regimens within a period of nine months. Data on ART medicines regimen that was routinely collected from ART pharmacies was analyzed and the resulting information shared among decision makers who were able to issue supplementary guidelines to correct an irrational and outdated ART prescribing practice.
A taskforce was formed under the leadership of the Ethiopian Federal Ministry of Health and it produced supplementary guidelines for managing the transfer of patients currently on stavudine to non-stavudine-containing regimens within a period of nine months. Data on ART medicines regimen that was routinely collected from ART pharmacies was analyzed and the resulting information shared among decision makers who were able to issue supplementary guidelines to correct an irrational and outdated ART prescribing practice.

