Background
Zambia is a sub-Saharan country with an adult HIV prevalence of 14.3%. Tuberculosis is the top cause of morbidity and mortality, especially among young and economically productive adults aged 15–49.[1] SIAPS, with support from USAID, implemented the following activities in Zambia.
Project Highlights
- In collaboration with Harvard Pilgrim Health Care, SIAPS piloted the introduction of the Intervention Guide for the Management of Childhood Illnesses in three districts in Zambia.
- Technical assistance provided for an early warning system (EWS) and the implementation of QuanTB, which is now updated bimonthly and used to track TB medicine stock, plan shipments, and take actions to avoid stock-outs and wastages. This information has resulted in the ability of stakeholders to take quick action for supply planning.
- A rapid situation analysis of TB data and commodity management was conducted in five member states of the East Central, Southern Africa Health Community (ECSA-HC) countries: Malawi, Swaziland, Tanzania, Uganda, and Zambia.
- Technical assistance provided for the development, revision, and dissemination of the essential medicine lists.
- Technical assistance was provided to build the capacity of the Ecumenical Pharmaceutical Network (EPN) to strengthening pharmaceutical services in church health systems. The EPN developed and conducted a training-of-trainers program on antimicrobial resistance (AMR) for member participants and, as a result, action plans to contain AMR at participants’ institutions were developed and implemented.
Results
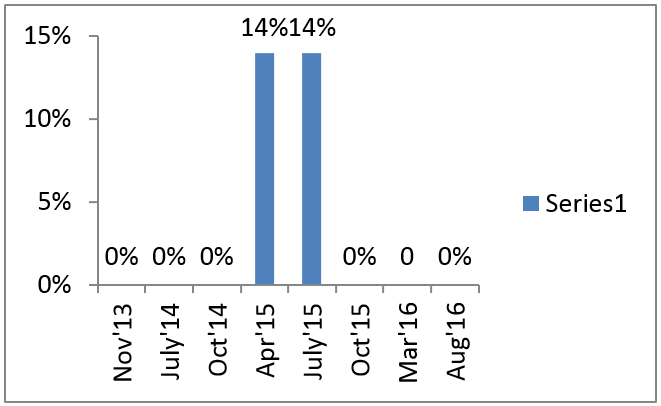
The ECSA-HC TB data and commodity management analysis showed that while there is a strong foundation for TB data and commodity management, improvements are needed, including the adoption and institutionalization of QuanTB and the EWS.
The adoption and institutionalization of QuanTB enhanced the quantification capacity and skills of the National TB Control Program (NTP). The trend of TB medicine stock-out rates improved, with no stock-outs of first-line TB medicines and almost no stock-outs of second-line TB medicines between November 2013 and April 2016. The 14% stock-out of second-line medicines between April and July 2015 was due to procedural delays in the delivery of an order from the Global Drug Facility, which have since been factored into the forecasting process.
Project Legacy
Based on the ECSA-HC member state study, the ECSA recommended the following:
- Establish a platform for TB commodities and information management for ECSA member states.
- Improve supply chain management of TB commodities.
- Strengthen human resource capacity for TB commodity management within the ECSA-HC.
- Strengthen TB lab commodities and data management among ECSA member states.
- Strengthen the NTP’s procurement and supply management department through adequate resource allocation, adopting an electronic logistics management information system, and introducing the delivery of first-line medicines at the district level to harmonize with the current medical stores limited system and reduce the length of the pipeline.
Resources
- Implementing QuanTB to Improve Forecasting, Supply Planning, and Early Warning Systems for TB Medicines: Zambia Report
- Standard Treatment Guidelines and Essential Medicines List
- Infection control assessment tool (ICAT)
[1] WHO Country Cooperation Strategy, 2008–2013. Available at: http://apps.who.int/iris/bitstream/10665/136150/1/ccs_zmb.pdf?ua=1

