Project Dates: September 2011 – March 2018
Background
The rates of TB, HIV, and AIDS in Swaziland are among the highest in the world. The HIV prevalence rate among 15 to 49 year olds in 2015 was 28.8%, and 80% of TB patients were co-infected with HIV (2014). The Government of Swaziland adopted a decentralization strategy to expand HIV treatment and care services, including the accreditation of private health facilities for the provision of antiretroviral therapy (ART). The scaled-up provision of antiretrovirals (ARVs) has put enormous pressure on the country’s pharmaceutical services, health sector budget, supply chain system, and human resources.
SIAPS started work in Swaziland in 2011 as a follow on to the Strengthening Pharmaceutical Systems program. Through SIAPS, USAID provided funding to Swaziland to support pharmaceutical system strengthening over a five-year period.
Project Highlights
The goal of USAID/SIAPS in Swaziland was to ensure the availability of quality pharmaceutical products and effective pharmaceutical services to achieve desired health outcomes for HIV/TB care and treatment.
Medicine Regulatory System
The legislation regulating medicines, pharmaceutical establishments, and the pharmacy profession in Swaziland was outdated and ineffective. SIAPS facilitated bills to establish the first ever Medicines Regulatory Authority (MRA) and the development of the 2012–2016 Pharmaceutical Strategic Plan to provide strategic direction for strengthening the pharmaceutical sector in Swaziland.
Human and Institutional Capacity Development
The shortage of qualified pharmacy personnel was a major limitation in the country’s efforts to expand HIV/AIDS and TB treatment programs.
Management of Information Systems for Better Patient Care
There was limited organizational and human resource capacity to implement a logistic management information system (LMIS) to ensure an uninterrupted supply chain for ARVs. SIAPS worked with in-country partners to revamp the LMIS and improve the use of RxSolution and other electronic tools for inventory and patient management.
Availability of Medicines
The rapid scale-up of HIV and TB treatment programs exposed weaknesses related to procurement and supply chain systems, indicated by frequent stock-outs of essential medicines. SIAPS supported the MOH to establish the Supply Chain Technical Working Group (TWG) to facilitate planning, procurement, and distribution to avoid both over- and under-stocking of key commodities.
Patient Safety and Treatment
To improve patient safety and promote adherence to HIV/TB treatment, SIAPS assisted the MOH to design interventions to address adverse drug reactions.
Results
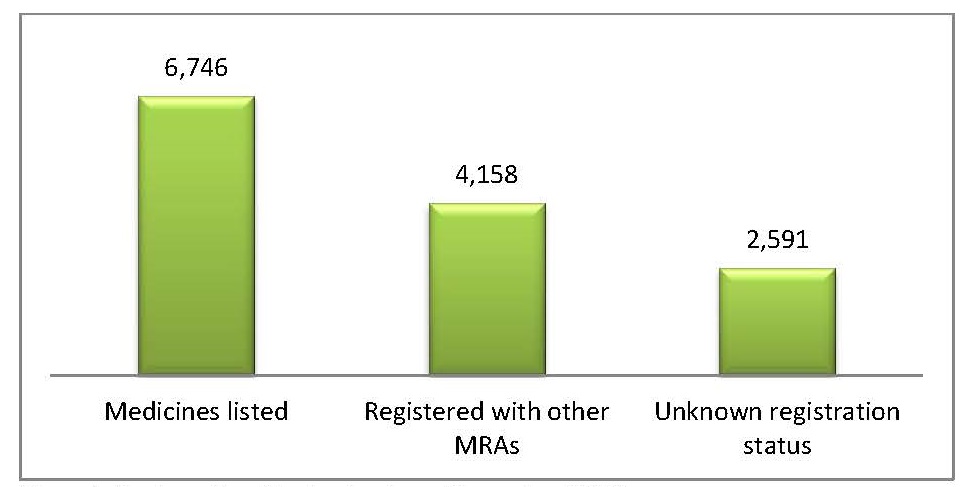
The Medicines and Related Substances Control Bill was signed into law in October 2016. The MOH can now register medicines and medicine importers and manage a National Drug Regulatory Authority. Swaziland has developed a database of medicine importers and the more than 6,000 products they import (figure 1).

Individual and Institutional Capacity to Deliver Pharmaceutical Services
With this new cadre of pharmacy workers, Swaziland has made progress in meeting its human resource needs to deliver high-quality pharmaceutical care and services. In July 2014, SANU graduated the first cohort of students with a certificate of pharmacy. By September 2016, 145 students had entered the program. Due to increasing demand, the certificate program was upgraded to a pharmacy degree in 2014.
Availability and Use of Data for Decision Making
The revised tools increased the health facility-level reporting rate from 56% in 2012 to 97% in 2014. By using LMIS data to resupply ARVs, 100% of facility orders were fulfilled and stock-outs of tracer ARVs were eliminated.
Stock-outs and the Availability of Medicines
As a result of coordination and better quantification systems and processes, the stock-out rate for tracer commodities decreased from 50% to 0% at the central warehouse and from 23% to 0% at health facilities from December 2011 to September 2014.

From June 2013 to September 30, 2016, 4,210 patients were enrolled in an active surveillance system and 1,224 adverse drug events (ADEs) were reported. Of the enrolled patients, 76% were taking ARVs and 24% were taking anti-TB medicines.
Project Legacy
Through advocacy and technical support, SIAPS played a crucial role in establishing the MRA, which will facilitate access to safe, effective, and quality medicines for the people of Swaziland. With the new cadre of pharmacy workers entering the workforce, Swaziland has taken an important step toward meeting its human resources needs to deliver high-quality pharmaceutical care and services.