Background
In 2015, Kenya had an estimated population of 46,050,000.[1] Approximately 81,518 cases of TB[2] and 1,499,027 cases of malaria were notified at health facilities.[3] The country’s maternal mortality rate is estimated to be 510/100,000 live births.[4] The uninterrupted availability of safe and effective commodities for the treatment and prevention of malaria and HIV/AIDS at various points of service delivery is fundamental to controlling disease-related mortality and morbidity. As in most endemic countries, malaria and TB commodities in Kenya are financed by donors. Insufficient funding for distribution costs; inaccurate forecasting, quantification, inventory management, and supply planning; and inadequate fund allocation are among the many factors that lead to stock-outs at health facilities.
Project Highlights
In Kenya, SIAPS has undertaken following activities:
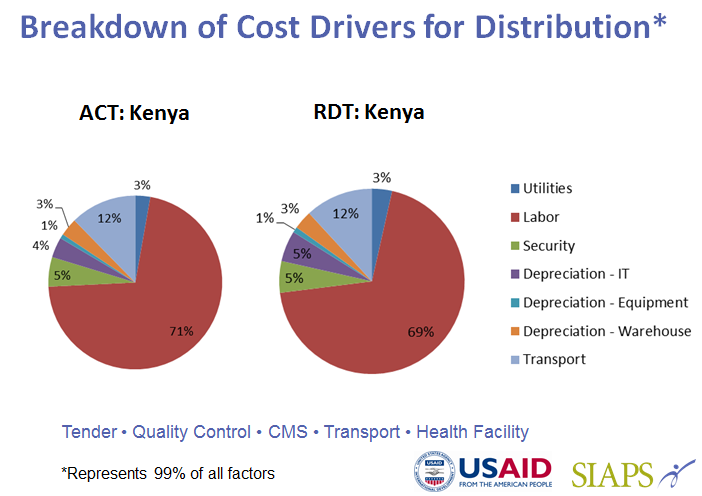
- Estimating the distribution costs of artemisinin-based combination therapies (ACTs) and rapid diagnostic tests (RDTs) from the central to the peripheral levels of the public sector health system and to community health workers in Kenya
- Assessing subnational procurement practices of maternal, newborn, and child health (MNCH) commodities
- Mapping the financial flow for MNCH commodities in the public sector
- Training national TB control program (NTP) drug management staff and partners to use QuanTB for quantifying and tracking TB medicines
Results
The assessment of subnational procurement practices for MNCH medicines found that the availability of those medicines varies depending on the county and is limited at both the lower levels of the system and tertiary care hospitals. Key findings include:
- A lack of guidelines for developing medicines budgets
- The approved and allocated budget is often less than requested
- There is limited availability and visibility of logistics data for decision making
- Funds are often not disbursed on time, leading to delays in payment of suppliers
- Monitoring expenditures for medicines are limited and not done at the subcounty level
- There is a lack of coordination between the national and county levels and among counties
SIAPS supported the NTP to determine the funding gap for TB medicines as a result of decentralization to counties. SIAPS also supported the NTP’s development of a new procurement and supply management component of the new strategic plan. SIAPS has worked with NTPs to improve oversight of the private sector and increase its potential to contribute to national TB management efforts. SIAPS finalized and published Drug Use Reviews: A Practical Strategy to Ensure the Rational Use of Anti- Tuberculosis Medicines and provided training to NTP staff in Kenya to pilot the program.
SIAPS provided technical assistance to Kenya’s NTP and Ministry of Health to implement new medicines and their regimens and to train NTP staff and nearly 500 health care workers. SIAPS provided support to the Global Drug Facility (GDF) and accompanied a GDF mission/joint program review in Kenya.
SIAPS worked with the Ecumenical Pharmaceutical Network (EPN) to support the management of results-oriented proposals from EPN’s member organizations on topics related to antimicrobial stewardship and antimicrobial resistance (AMR). Gertrude’s Children’s Hospital conducted an assessment of staff adherence to standard treatment guidelines (STGs) and, based on the findings, trainings were conducted for physicians and pharmacy staff on the appropriate use of STGs and their impact on AMR.
Legacy
Kenya’s National Tuberculosis, Leprosy and Lung Disease Program has adopted and fully institutionalized QuanTB as the national quantification tool for TB medicines. The quantification results using QuanTB are more timely, accurate, and reliable than were those prior to QuanTB. Quantities for procurement are easily determined, and orders are placed to ensure an uninterrupted supply of TB medicines. Because of Kenya’s decision to implement a decentralized system, the findings from the subnational procurement of MNCH medicines and the financial flow will help local governments implement strategies to strengthen county health systems.
Resources
- Estimating the In-Country Distribution Costs of Malaria Commodities in Benin and Kenya
- Implementing QuanTB to Improve Forecasting, Supply Planning, and Early Warning Systems for TB Medicines: Kenya Report
- Other Documents
[1] WHO; Kenya country statistics
[2] https://extranet.who.int/sree/Reports?op=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=KE&outtype=html
[3] http://www.who.int/malaria/publications/country-profiles/profile_ken_en.pdf?ua=1
[4] http://www.who.int/gho/maternal_health/countries/ken.pdf?ua=1

