Project dates: September 2011–March 2017
Background
Ethiopia’s major health challenges include preventable communicable diseases and nutrition disorders. More than 90% of child deaths are due to pneumonia, diarrhea, malaria, neonatal problems, malnutrition, HIV/AIDS, or a combination. The maternal mortality ratio has declined to 470/100,000 but remains among the world’s highest. Pharmaceutical management challenges included irrational medicine use and poor prescribing practices.
SIAPS began working in Ethiopia in 2011 and over five years, USAID provided USD 17.5 million to support pharmaceutical systems strengthening. SIAPS partnered with key Ethiopian institutions to alleviate these challenges by implementing interventions that contribute to PEPFAR, PMI, USAID, and the Government of Ethiopia’s common goal of achieving an “AIDS-free Generation” (90-90-90) and reducing preventable malaria-related morbidity and mortality and maternal/child deaths.
Project highlights
– Strengthening Governance
SIAPS supported government stakeholders to implement regulations, policies, guidelines, and SOPs that govern the pharmaceutical sector:
– Improving Service Delivery
Pharmaceutical services and pharmacovigilance: To improve medicine availability and quality of care, SIAPS supported legislation for auditable pharmaceuticals transactions and services (APTS) in hospitals. SIAPS designed the Pharmacovigilance Data Management System, which has resulted in efficient reporting of pharmacovigilance data at the FMHACA.
Clinical services: SIAPS supported the development and implementation of an in-service training program for hospital pharmacists.
Management of antimalarials: A medicine management handbook was developed for health extension workers for the management of antimalarials and other essential medicines. The Continuous Results Monitoring System (CRMS)was implemented to track the availability, storage, and use of antimalarials.
Individual and institutional capacity: SIAPS focused on gaps in government priorities related to human capital and institutional preparedness to provide quality, sustainable pharmaceutical services.
Information for decision making: SIAPS Ethiopia designed and implemented information systems to address disease-specific and overall health system strengthening needs.
Financing for medicines: SIAPS built the capacity of health facilities to achieve cost savings during the selection and prioritization of medicines for procurement.
Results
Achievements include:
– The information center at the FMHACA has improved transparency and access to information by clients and the public

– APTS regulations have institutionalized critical interventions that have created accountability and ensured sustainability of system strengthening interventions.
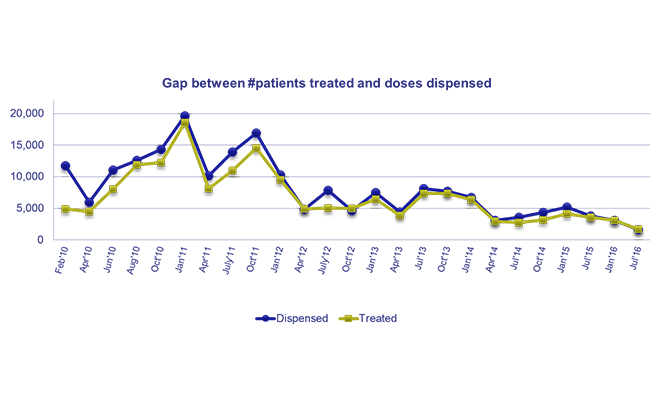
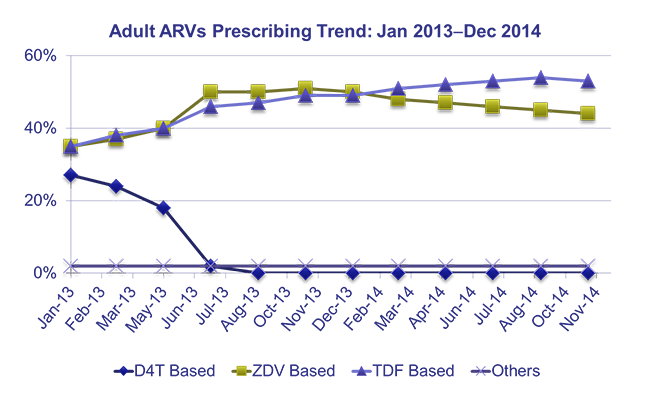
– The National Advisory Committee developed a national strategy to prevent and contain AMR and guide the implementation of strategies to minimize the risk of AMR. In addition, DTCs own and lead the implementation of facility-level interventions, including rational medicine use and antimicrobial resistance activities.
– Between 2012 and 2014, 200 pharmacists from 65 hospitals were trained and deployed, and 98.5% provided clinical pharmacy services. A study of 43 hospitals found that 17 (39.5%) had full-time pharmacists in their wards supporting clinical services in 36 hospitals and identifying and supporting the management of 8,257 drug therapy problems.
– A total of 7,720 professionals were trained, and SOPs have standardized pharmacy practice across facilities. Streamlining the supply chain of medicines and rationalizing treatment for common conditions has enhanced the availability of medicines in public facilities.
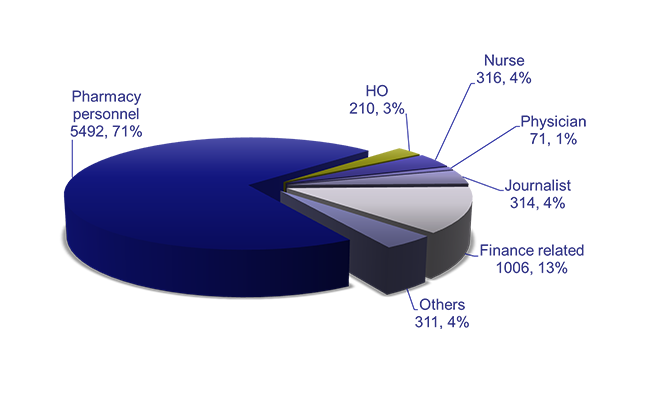
– The first patient-centered service delivery model for pharmacy services in Ethiopia was introduced.
– Pharmacovigilance and antimicrobial resistance courses were introduced into university curricula and integrated into preservice education.