Project dates: October 2011–December 2016
Background
The Democratic Republic of the Congo (DRC) is the second largest country in Africa, with an area of 2,345,409 square kilometers and an estimated population of 85 million (National Institute of Statistics 2014). It is characterized by a fast-growing population (2.7% per year) and a fertility rate of 5.9 children per woman (WHO statistical profile 2012).
The country’s pharmaceutical challenges include:
- A growing number of illegal and uncontrolled pharmaceutical businesses
- Circulation of counterfeit and substandard medicines in the country
- Unreliable information systems, a shortage of trained and qualified personnel, and poor coordination of key stakeholders, resulting in poor availability of medicines in the country
Between October 2011 and December 31, 2016, SIAPS provided technical assistance to the Ministry of Health (MOH), Drug Regulatory Authority (DRA), National Medicine Supply Program (PNAM), national disease programs, and provincial health divisions (DPS) to improve the availability of medicines.
Project Highlights
Results
Governance and Leadership
- A Medicine Registration Committee was established that has streamlined the registration and regulation of medicines in DRC. The number of registered medicines increased from 200 in 2010 to more than 4,600 in December 2016.
- Of the medicines currently included in the National Essential Medicines List, 72% have at least one product registered, an increase from 44% in 2011 (figure 1).
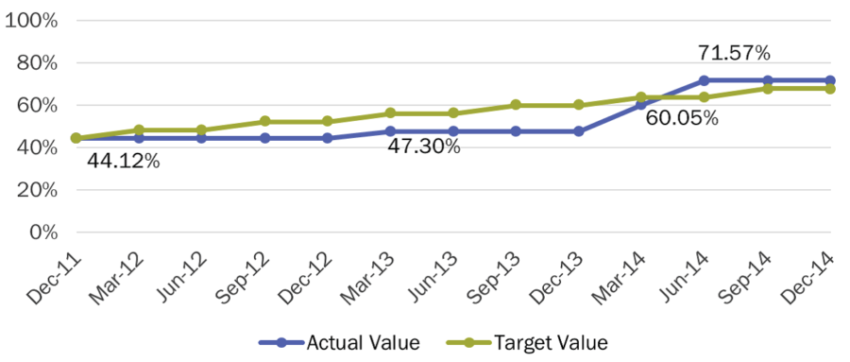
- The number of days to process a new application decreased from 82 in 2013 to 58 in 2016.
- Therapeutic committees have been deployed at the provincial and hospital levels to ensure rational medicine use.
- Thirteen pharmaceutical management guidelines, lists, and SOPs were developed or updated and submitted for adoption, including:
-The National Essential Medicine List
-Registered medicines directory
-Standards treatment guidelines
-Standards for the use of oxytocin, misoprostol, and chlorhexidine digluconate
-Maternal, newborn, and child health fact sheets
Stock Availability at the Facility Level
- By 2016, 75% of facilities used a standardized checklist to monitor storage conditions, an increase from 0% in December 2011.
- The percentage of SIAPS-supported health facilities and warehouses with stock-outs of a preselected group of medicines for three days or more in the previous three months decreased from 100% in December 2011 to 36% (health facilities) and 13% (warehouses) in July 2016.
- SIAPS assisted the Regional Procurement Association for Essential Medicines (ASRAMES), the largest warehouse in the eastern part of DRC, to meet the USAID/European Union prequalification standards. ASRAMES is used as a local supplier to address the long lead time needed when importing pharmaceutical products.
Institutionalizing a Training Curriculum to Meet Current Public Health Needs
- FOPS became the first training institution in DRC to have a strategic plan and is the only one that meets World Bank and other donor requirements for future funding.
Pharmaceutical Service Delivery
- The CNPV receives approximately 1,500 individual case safety reports per year with a completeness score (> 75%), which is well above the average score for other countries (50%).
- CNPV staff support the management of adverse events at the point of care.
Project Legacy
During the five years of SIAPS, DRC has built the institutional and individual capacities of the DPM, PNAM, and CNPV; ensured appropriate preservice training for future pharmacy professionals; and strengthened the medicine distribution and data collection and reporting processes. The project succeeded in strengthening governance in the DRC pharmaceutical sector by enabling proper medicine registration at the DPM and producing a national list of registered medicines thereby improving the safety and availability of medicines, including antiretrovirals.
Resources
- Strengthening Regulatory Systems to Improve Access to Safe, Effective, and Quality Medicines
- Analysis of Bottlenecks Related to Demand, Supply, and Use of Antibiotics for the Treatment of Neonatal Sepsis in the DRC
- Implementing QuanTB to Improve Forecasting, Supply Planning, and Early Warning Systems for TB Medicines: Report
- Other Documents