
The purpose of this standard operating procedure is to outline a step-by-step approach for undertaking active drug safety monitoring for the 9-month MDR-TB treatment regimen and other novel medications and regimens for TB and MDR-TB.

The purpose of this standard operating procedure is to outline a step-by-step approach for undertaking active drug safety monitoring for the 9-month MDR-TB treatment regimen and other novel medications and regimens for TB and MDR-TB.
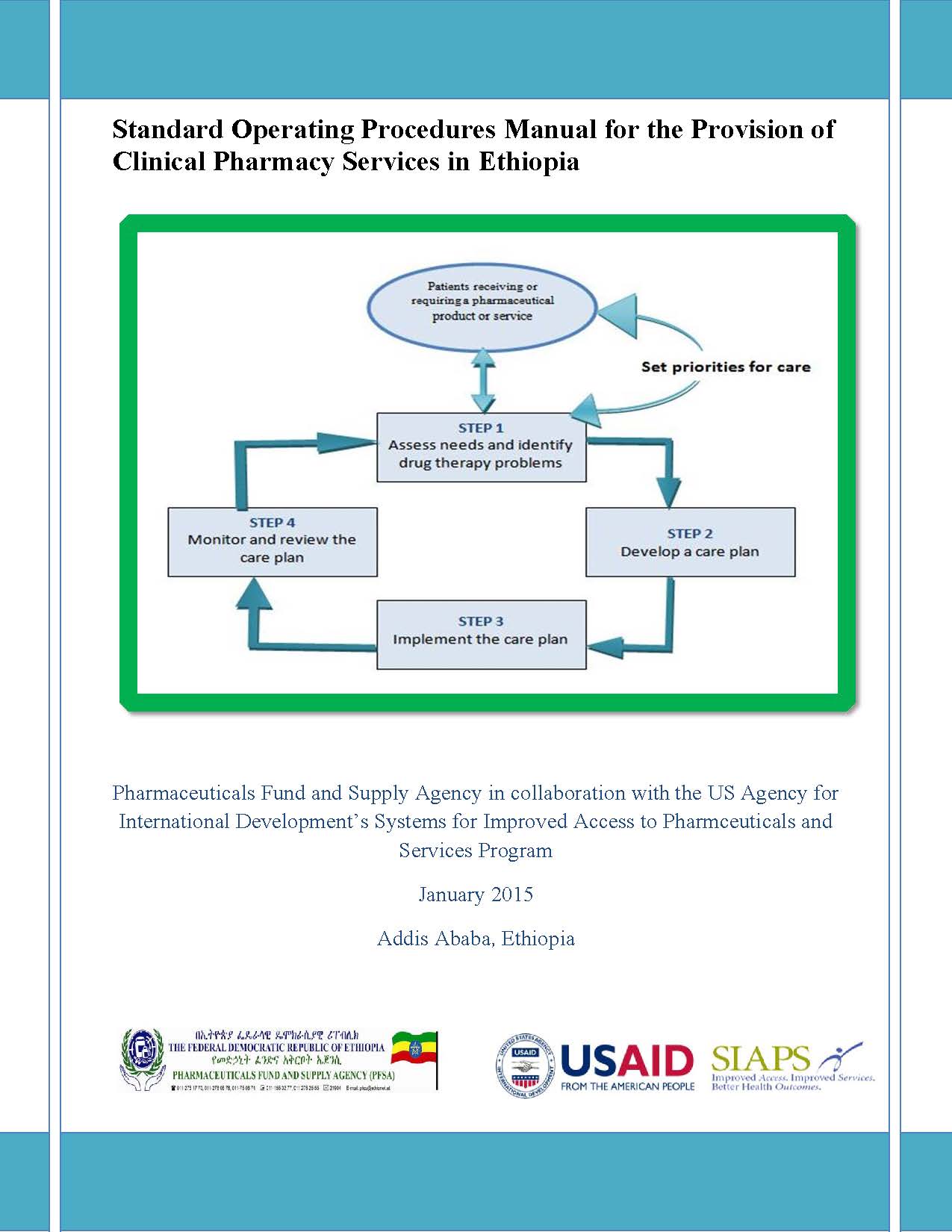
The direct involvement of pharmacists in patient care (clinical pharmacy services) is a key intervention to optimize the outcomes of medicine therapy, thereby improving the quality of patient care. The Pharmaceuticals Fund and Supply Agency (PFSA) has been collaborating in efforts to implement clinical pharmacy services in the Ethiopian health care system. As part of … Read more

This document provides a summary of the SIAPS project in the Dominican Republic; including background information on the project, USAID support, and achievements of USAID and its partners.