
This infographic provides an overview of select SIAPS interventions and results in line with six core health system functions: governance; capacity building; information for decision-making; financing; supply chain; and pharmaceutical services.

This infographic provides an overview of select SIAPS interventions and results in line with six core health system functions: governance; capacity building; information for decision-making; financing; supply chain; and pharmaceutical services.
To take systems strengthening support to the next level, SIAPS is introducing a web-based enhanced information graphic display platform. The dashboard features data from each health facility and supply structure and will provide real-time access to patient and commodity information. The end goal is for the dashboard to be used to visualize graphic data on health programs, … Read more

Patients on lifelong antiretroviral therapy (ART) may be at risk of forgetting their pharmacy appointments for antiretroviral (ARV) refills. The SMS-based ART pharmacy appointment and medication adherence reminder service provides automated text notifications to ART patients reminding them to pick up their ARV refills according to appointment dates made by pharmacy staff using the SIAPS-supported Electronic … Read more

Swaziland, working to combat two concurrent epidemics of HIV and tuberculosis (TB), has recently intensified nationwide HIV testing and TB case finding campaigns. However, these efforts have been hampered by routine stock-outs of key TB, laboratory, HIV and AIDS, and other health commodities. In December 2011, 50% of tracer products were stocked out at the central level, while 23% of tracer products were … Read more

SIAPS’ predecessor project, Strengthening Pharmaceutical Systems, worked with the government to establish the SUGEMI system to provide more accurate information on consumption, forecasting, pricing, and distribution. SUGEMI is an information system that is fully aligned with the country’s health sector reform process, compatible with the decentralized health sector, and designed to coordinate information across different vertical disease programs. These features were essential to promote the … Read more

Over the past three years, SIAPS has expanded the use of RxSolution to a total of 350 sites across South Africa. SIAPS is also developing numerous customized modules and modifications to meet the specialized needs of different clinical settings including expanded functionality for barcode tracking, biometric monitoring, and mobile phone applications.

SIAPS is working to help governments, hospitals, and health facilities better manage data by strengthening the systems that support effective pharmaceutical information management. In addition to improving the collection, quality, and analysis of data, SIAPS is working in partnership with local stakeholders to develop informational dashboards which highlight and make available the key pieces of data needed for effective decision making.
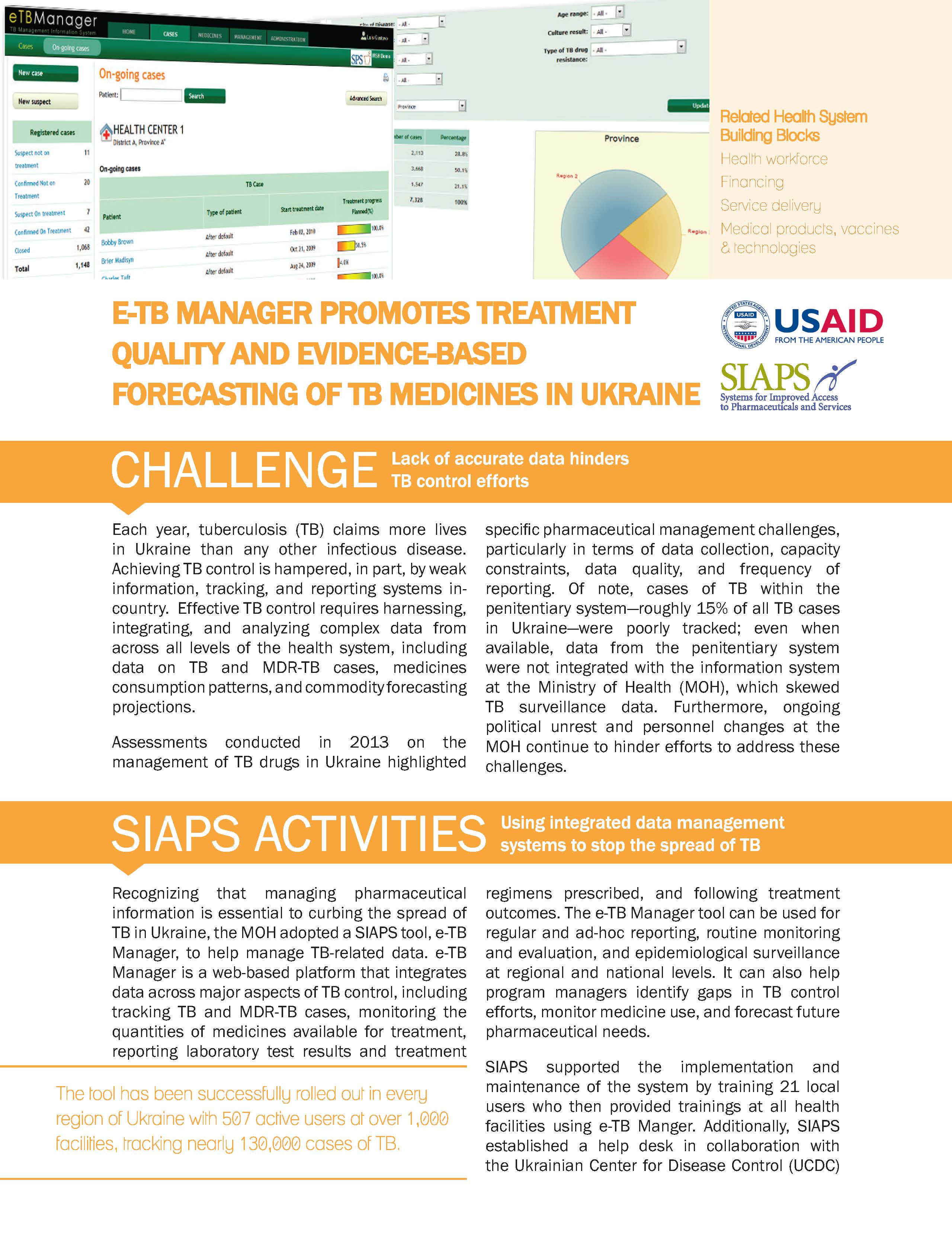
Assessments conducted in 2013 on the management of TB drugs in Ukraine highlighted specific pharmaceutical management challenges, particularly in terms of data collection, capacity constraints, data quality, and frequency of reporting. Of note, cases of TB within the penitentiary system―roughly 15% of all TB cases in Ukraine―were poorly tracked; even when available, data from the penitentiary system were not integrated with the information system at … Read more

The weak regulatory system in Swaziland poses a threat to public health and safety and has been an obstacle in the country’s effort to improve access to quality essential medicines and services for managing HIV, controlling tuberculosis, and delivering other priority public health interventions. The legislation governing the regulation of medicines, pharmaceutical establishments, and the pharmacy profession in Swaziland dates back to 1929 and … Read more

The purpose of this document is to provide guidance to program managers in ministries of health at national and sub-national levels as well as personnel in other interested organizations on actions to take and factors to consider when expanding access to essential RMNCH commodities. While this document focuses on RMNCH medicines and supplies, it may be used as a … Read more