The main purpose of this inventory is to serve as a reference to help stakeholders working in the pharmaceutical sector easily access and use already available SIAPS resources, including tools, experiences, and results. The document is also intended to serve as a technical legacy for SIAPS to support knowledge exchange and sustainability of related work. … Read more
AMR, Antimicrobial Resistance, Drug and Therapeutics Committee, essential medicines list, HIV/AIDS, Malaria., neglected tropical disease, pharmaceutical financing, pharmaceutical services, rational medicine use, RMNCH, standard treatment guidelines, Supply chain management, tuberculosis
The Systems for Improving Access to Pharmaceuticals and Services (SIAPS) Program Mozambique has been working with DFH and partners in the pharmaceutical sector and in priority health programs to assist pharmaceutical services in improving the availability of pharmaceutical products and appropriate use at the service delivery points with the aim of achieving desired health outcomes. … Read more
The WHO estimates that more than 50% of all medicines are prescribed, dispensed or sold inappropriately, and that 50% of all patients fail to take them correctly. The overuse, underuse or misuse of medicines—also known as irrational use of medicines—results in wastage of scarce resources, increased risk of adverse drug reactions, and widespread health hazards, … Read more
This document provides a background and basic guidance to medicine use data collection to be undertaken by University of Namibia School of Pharmacy second-year students during their placement in rural health facilities. In 2012, UNAM-SoP introduced 4-week placements at health facilities located in rural communities for pharmacy students as part of their practical training. This … Read more
A new manual published by the USAID-funded Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program aims to guide health care professionals in the development and implementation of standard treatment guidelines (STGs). STGs are designed to assist health care professionals in making decisions about appropriate, effective patient care. However, health managers often have trouble … Read more
STGs are designed to assist health care professionals in making decisions about appropriate, effective patient care. However, health managers often have trouble setting and meeting the high standards required of modern, developed health care systems. With stakeholders expressing concern over issues such as strength of evidence, transparency, conflicts of interest, and effective implementation, it is … Read more
SIAPS and its predecessor projects—Strengthening Pharmaceutical Systems (SPS) and Rational Pharmaceutical Management (RPM) Plus—have worked extensively to introduce and support tools that capture dispensing and supply chain data from public health facilities. SIAPS supports the Ministry of Health and Social Services (MoHSS) to conduct periodic analyses of antiretroviral therapy (ART) data by using available data … Read more
Enhancing patient recordkeeping in Tshwane Metropolitan Municipality The City of Tshwane Metropolitan Municipality, located in the Gauteng Province, is the fifth-largest municipality in South Africa and home to over 2.9 million residents as well as the capital, Pretoria. The municipality operates 24 primary health care facilities providing access to health services, primarily to the uninsured … Read more
A year ago, the World Health Organization (WHO) released its first global report on antimicrobial resistance, revealing a number of troubling trends: rising rates of resistance to first-line antibiotics for common infections such as urinary tract infections, gonorrhoea, and staph infections; a growing inability to treat deadly diseases like tuberculosis (TB) with second-line and even … Read more
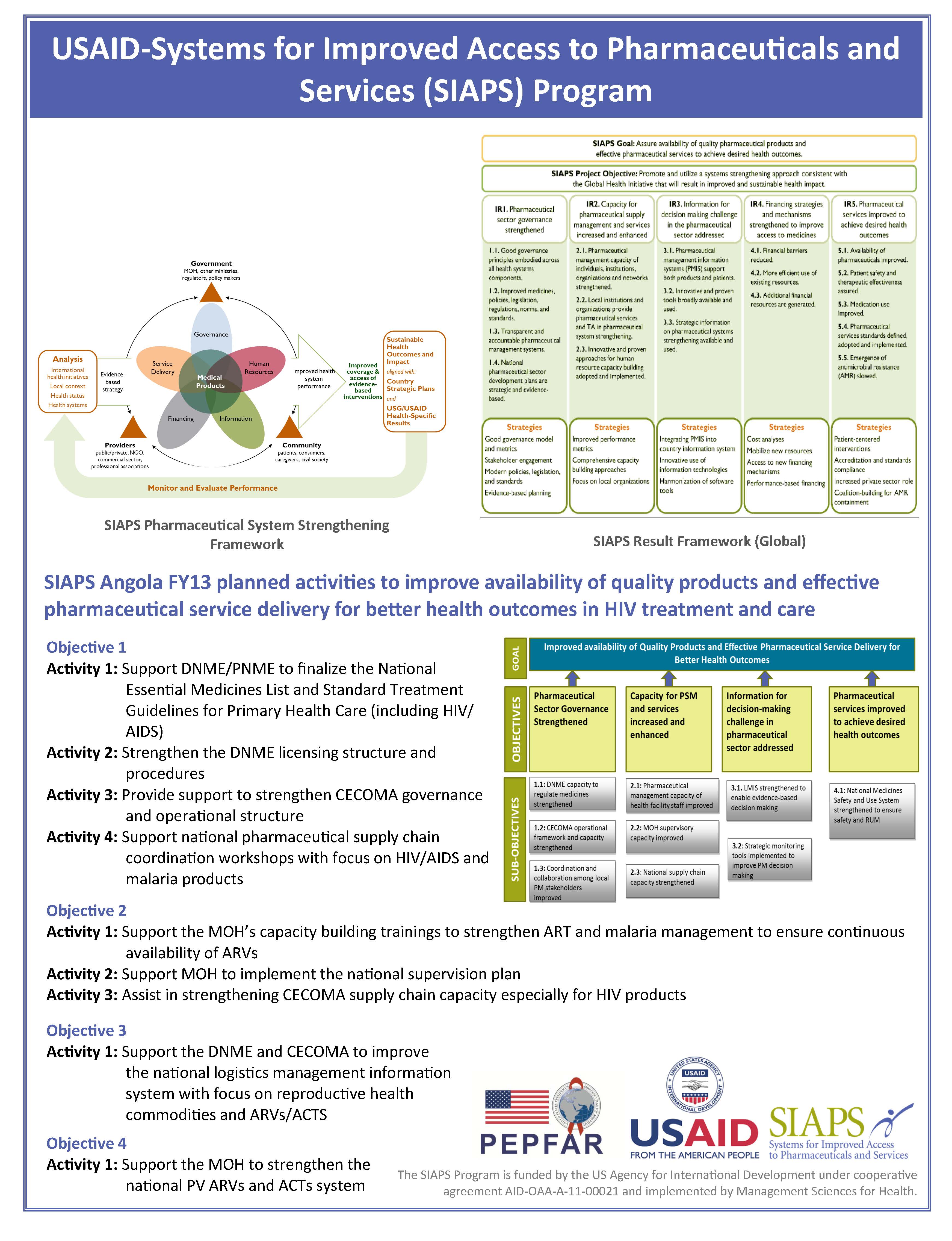
SIAPS Angola FY13 planned activities to improve availability of quality products and effective pharmaceutical service delivery for better health outcomes in HIV treatment and care